1. Eggermann T, Venghaus A, Zerres K. Cystinuria: an inborn cause of urolithiasis. Orphanet J Rare Dis. 2012; 7:19.
2. Palacín M, Borsani G, Sebastio G. The molecular bases of cystinuria and lysinuric protein intolerance. Curr Opin Genet Dev. 2001; 11:328–335.
3. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006; 367:333–344.
4. Knoll T, Zöllner A, Wendt-Nordahl G, Michel MS, Alken P. Cystinuria in childhood and adolescence: recommendations for diagnosis, treatment, and follow-up. Pediatr Nephrol. 2005; 20:19–24.
5. Claes DJ, Jackson E. Cystinuria: mechanisms and management. Pediatr Nephrol. 2012; 27:2031–2038.
6. Shim M, Park HK. Multimodal treatments of cystine stones: an observational, retrospective single-center analysis of 14 cases. Korean J Urol. 2014; 55:515–519.
7. Rosenberg LE, Downing S, Durant JL, Segal S. Cystinuria: biochemical evidence for three genetically distinct diseases. J Clin Invest. 1966; 45:365–371.
8. Dello Strologo L, Pras E, Pontesilli C, Beccia E, Ricci-Barbini V, de Sanctis L, Ponzone A, Gallucci M, Bisceglia L, Zelante L, et al. Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: a need for a new classification. J Am Soc Nephrol. 2002; 13:2547–2553.
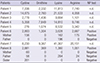
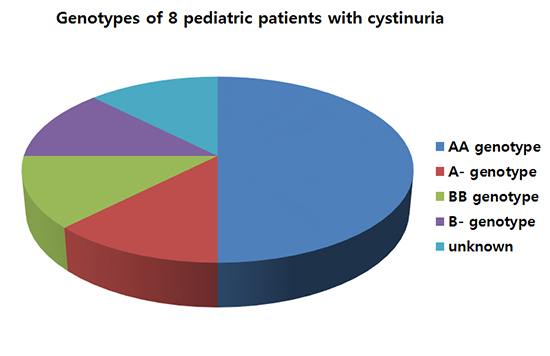
9. Choi JE, Yun BY, Park HW, Park JH, Ha IS, Jeong HI, Choi Y, Choi H, Kim IW. Cystinuria 3 cases. J Korean Pediatr Soc. 1995; 38:245–251.
10. Kang EG, Lee JH, Lee BH, Kim GH, Park YS. A case of cystinuria with multiple renal stones in an 8-month-old girl. J Korean Soc Pediatr Nephrol. 2013; 17:122–126.
11. Lee EH, Kim YH, Hwang JS, Kim SH. Non-type I cystinuria associated with mental retardation and ataxia in a Korean boy with a new missence mutation(G173R) in the SLC7A9 gene. J Korean Med Sci. 2010; 25:172–175.
12. Lee ST, Cho H. Metabolic features and renal outcomes of urolithiasis in children. Ren Fail. 2016; 38:927–932.
13. Cho CH, Hahm KS, Park JK, Kim KH. A case of cystine stone in a child. Korean J Urol. 1986; 27:933–938.
14. Hah ST, Yoon JH, Yoon JB. Cystine stone: report of two cases. Korean J Urol. 1981; 22:451–455.
15. Wong KA, Mein R, Wass M, Flinter F, Pardy C, Bultitude M, Thomas K. The genetic diversity of cystinuria in a UK population of patients. BJU Int. 2015; 116:109–116.
16. Rhodes HL, Yarram-Smith L, Rice SJ, Tabaksert A, Edwards N, Hartley A, Woodward MN, Smithson SL, Tomson C, Welsh GI, et al. Clinical and genetic analysis of patients with cystinuria in the United Kingdom. Clin J Am Soc Nephrol. 2015; 10:1235–1245.
17. Saadi I, Chen XZ, Hediger M, Ong P, Pereira P, Goodyer P, Rozen R. Molecular genetics of cystinuria: mutation analysis of SLC3A1 and evidence for another gene in type I (silent) phenotype. Kidney Int. 1998; 54:48–55.
18. Schmidt C, Vester U, Hesse A, Lahme S, Lang F, Zerres K, Eggermann T. Arbeitsgemeinschaft Pädiatrische Nephrologie. The population-specific distribution and frequencies of genomic variants in the SLC3A1 and SLC7A9 genes and their application in molecular genetic testing of cystinuria. Urol Res. 2004; 32:75–78.
19. Popovska-Jankovic K, Tasic V, Bogdanovic R, Miljkovic P, Golubovic E, Soylu A, Saraga M, Pavicevic S, Baskin E, Akil I, et al. Molecular characterization of cystinuria in south-eastern European countries. Urolithiasis. 2013; 41:21–30.
20. Albers A, Lahme S, Wagner C, Kaiser P, Zerres K, Capasso G, Pica A, Palacin M, Lang F, Bichler KH, et al. Mutations in the SLC3A1 gene in cystinuric patients: frequencies and identification of a novel mutation. Genet Test. 1999; 3:227–231.
21. Yuen YP, Lam CW, Lai CK, Tong SF, Li PS, Tam S, Kwan EY, Chan SY, Tsang WK, Chan KY, et al. Heterogeneous mutations in the SLC3A1 and SLC7A9 genes in Chinese patients with cystinuria. Kidney Int. 2006; 69:123–128.
22. Sakamoto S, Cheong HI, Naya Y, Shigeta Y, Fujimura M, Ueda T, Mikami K, Akakura K, Masai M, Ichikawa T. Genomic characteristics of Asian cystinuria patients. Eur Urol Suppl. 2014; 13:e806.
23. Assimos DG, Leslie SW, Ng C, Streem SB, Hart LJ. The impact of cystinuria on renal function. J Urol. 2002; 168:27–30.
24. Worcester EM, Parks JH, Evan AP, Coe FL. Renal function in patients with nephrolithiasis. J Urol. 2006; 176:600–603.
25. Worcester EM, Coe FL, Evan AP, Parks JH. Reduced renal function and benefits of treatment in cystinuria vs other forms of nephrolithiasis. BJU Int. 2006; 97:1285–1290.
26. Gambaro G, Favaro S, D’Angelo A. Risk for renal failure in nephrolithiasis. Am J Kidney Dis. 2001; 37:233–243.
27. Prot-Bertoye C, Lebbah S, Daudon M, Tostivint I, Bataille P, Bridoux F, Brignon P, Choquenet C, Cochat P, Combe C, et al. CKD and its risk factors among patients with cystinuria. Clin J Am Soc Nephrol. 2015; 10:842–851.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download