INTRODUCTION
MATERIALS AND METHODS
Construction and preparation of recombinant attenuated S. typhimurium strains TP and TPH
Transfection of human GES-1 cells by recombinant attenuated S. typhimurium strains
Detection of expression level of HGF mRNA by reverse transcription–-polymerase chain reaction (RT-PCR)
Detection of expression level of HGF protein by enzyme-linked immunosorbent assay (ELISA)
Evaluation of the HGF activity in GES-1 cells by MTT method
Effect of the HGF expression product on the angiopoiesis of chick chorioallantois
Induction and treatment of rat gastric ulcer model
Gross and histological observation and scoring
Immunohistochemical staining for microvascular density (MVD) with CD34 expression
Detection of HGF and c-Met expression in gastric mucosa by ELISA
RESULTS
High expression of HGF in the GES-1 cells transfected with TPH in vitro
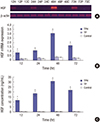 | Fig. 1HGF expression in the GES-1 cells after transfection with TPH or TP at different time points.
(A) RT-PCR products of HGF and β-actin in the agar gel electrophoresis. (B) The statistical results of RT-PCR of HGF in the GES-1 cells. TPH group: 3 × 107cfu TPH strain was added into the wells of a 6-well plate; TP group: 3 × 107cfu TP strain was added into the wells of a 6-well plate; control group: PBS (10 μL/well) was added into the wells of a 6-well plate; 12, 24, 48, and 72 hours indicate transfected TPH or TP or PBS for 12, 24, 48, and 72 hours, respectively. (C) Results from an ELISA of HGF concentration in cell supernatants. Cell supernatants were collected at different times after transfection, and the HGF levels were detected by ELISA. TPH group: 3 × 107 cfu TPH strain was added into the wells of a 6-well plate; TP group: 3 × 107 cfu TP strain was added into the wells of a 6-well plate; control group: PBS (10 μL/well) was added into the wells of a 6-well plate; 12, 24, 48, and 72 hours indicate the HGF levels from supernatant of cells transfected by TPH or TP or PBS for 12, 24, 48, and 72 hours, respectively.
GES-1 = gastric epithelial cells, TPH = attenuated Salmonella typhimurium strain carrying a eukaryotic expression vector encodes the human HGF gene, TP = attenuated Salmonella typhimurium strain with a eukaryotic expression vector, HGF = hepatocyte growth factor, RT-PCR = reverse transcription-polymerase chain reaction, PBS = phosphate buffer solution, ELISA = enzyme-linked immunosorbent assay.
*P < 0.01, TPH group vs. TP and control groups.
|
Stimulating effect of HGF on GES-1 proliferation
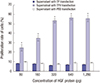 | Fig. 2TPH induced GES-1 cell proliferation.
TPH transfected the GES-1 cells. After incubation at 37°C for 48 hours, cell proliferation was measured by an MTT assay. TPH group: 3 × 103 cells/well (transfected by TPH strain) in a 96-well plate incubated at 37°C for 48 hours; TP group: 3 × 103 cells/well (transfected by TP strain) in a 96-well plate incubated at 37°C for 48 hours; control group: 3 × 103 cells/well (transfected by PBS) in a 96-well plate incubated at 37°C for 48 hours.
GES-1 = gastric epithelial cells, MTT = 2-(4,5-dimethyltriazol-2-yl)-2,5-diphenyl tetrazolium bromide, TPH = attenuated Salmonella typhimurium strain carrying a eukaryotic expression vector encodes the human HGF gene, TP = attenuated Salmonella typhimurium strain with a eukaryotic expression vector, PBS = phosphate buffer solution.
*P < 0.01, TPH group vs. TP and control groups.
|
Angiogenesis activity of HGF on the chick CAM
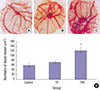 | Fig. 3Angiogenesis activity of the HGF expression product on the chick CAM.
(A) Control group: only a methylcellulose disk on CAM. (B) TP group: a disk with same volume supernatant from TP-transfected supernatant on CAM. (C) TPH group: a disk with the HGF expression product (300 pg) on CAM, from TPH-transfected supernatant. (D) The number of blood vessels was counted under a light microscope, and was found to be significantly fewer in expression supernatant from the TP-transfected and control groups than in the HGF expression product from the TPH-transfected group (P < 0.05).
HGF = hepatocyte growth factor, CAM = chorioallantoic membrane, TP = attenuated Salmonella typhimurium strain with a eukaryotic expression vector, TPH = attenuated Salmonella typhimurium strain carrying a eukaryotic expression vector encodes the human HGF gene.
*P < 0.05, TPH group vs. TP and control groups.
|
Gastric ulcer healing with TPH treatment
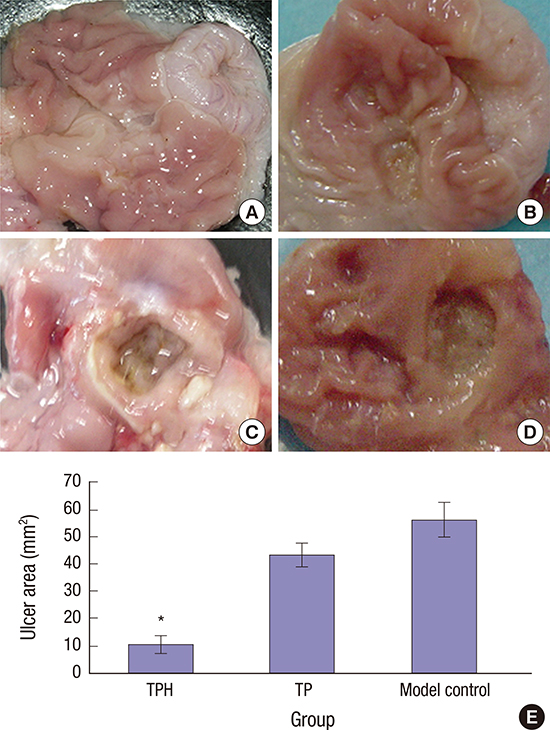
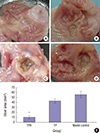 | Fig. 4Gross observation of gastric mucosa on day 21 after induced ulcer treatment.
(A) Normal control group: the gastric mucosae from normal rats were smooth and intact with no ulceration. (B) TPH treatment group: treated with (1 × 109) cfu TPH for acetic acid–induced gastric ulcer rats by gavage, the gastric mucosae of the TPH-treatment group were similar to the normal rats. The gastric mucosal lesions were smaller and shallow, and the regenerative granulation tissues were observed in ulcers. (C) Model control group: treated with 0.5-mL1.19M NaHCO3 for acetic acid–induced gastric ulcer rats by gavage. (D) TP treatment group: treated with 1 × 109 cfu TP for acetic acid–induced gastric ulcer rats by gavage. In the model control and TP groups, enlarged, deepened ulcers with severe adhesions to adjacent tissues were observed, the mucosa underwent necrosis, ulcerations, and had a dark yellowish membrane-like coating. The adjacent mucosa had obvious hyperemia and edema. (E) The statistical results of the mean ulcer area size (mm2). The size of the ulcer area in 21 days after TPH treatment was smaller than that in the other two groups.
TPH = attenuated Salmonella typhimurium strain carrying a eukaryotic expression vector encodes the human HGF gene, TP = attenuated Salmonella typhimurium strain with a eukaryotic expression vector.
*P < 0.01, TPH group vs. TP and control groups.
|
Histological assessment
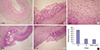 | Fig. 5Histological observations of the gastric mucosa.
(A) Normal control group: Clear glands and intact mucosa with abundant submucosal vessels were observed (H&E stain × 100). (B) TPH treatment group: Increased number of glands and submucosal granulation tissue regeneration were observed in the bases of ulcers along with regenerated capillaries. The gastric mucosa significantly healed (H&E stain × 100). (C) TPH treatment group: Increased number of glands and submucosal granulation tissue regeneration were observed in the bases of ulcers along with regenerated capillaries. The gastric mucosa significantly healed (H&E stain × 400). (D) TP-treated group: Severe mucosal anabrosis and depletion with ulceration were observed. The reepithelialization of gastric mucosa increased in the TP-treated group than in the model control group; however, no significant histological difference was found between the TP and the model control groups (H&E stain × 100). (E) Model control group: Severe mucosal anabrosis and depletion with ulceration were observed (H&E stain × 100). (F) The histological scoring for the crawling length of gastric epithelial cells from three groups. After treatment with TPH, the crawling length of gastric epithelial cells was significantly longer than in the TP and model control groups (P < 0.05). No statistical significance was observed in the mucosal epithelial crawling length between the TP and the model control groups after treatments (P > 0.05).
H&E stain = hematoxylin-eosin stain, TPH = attenuated Salmonella typhimurium strain carrying a eukaryotic expression vector encodes the human HGF gene, TP = attenuated Salmonella typhimurium strain with a eukaryotic expression vector.
*P < 0.01, TPH group vs. TP and control groups.
|
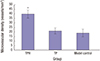 | Fig. 6Quantification of MVD on day 21 in granulation tissue from the ulcer beds by immunoreaction with CD34.
Data are presented as mean ± standard error (n = 50). The MVD in the TPH-treated group increased compared to the TP (a recombinant attenuated Salmonella strain carrying a eukaryotic expression vector)-treated and model control groups, with a significant statistical difference. The MVD in the TP-treated group was not significantly different compared with the model control group.
MVD = microvascular density, TPH = attenuated Salmonella typhimurium strain carrying a eukaryotic expression vector encodes the human HGF gene, TP = attenuated Salmonella typhimurium strain with a eukaryotic expression vector.
*P < 0.01, TPH group vs. TP and control groups.
|
Quantification of MVD in granulation tissue from the ulcer beds by immunoreaction with CD34
HGF and c-Met protein expression
 | Fig. 7HGF and c-Met protein expression in gastric mucosa by ELISA.
On day 21, the expression of HGF protein in gastric ulcer was significantly higher in the TPH-treated group than in the TP-treated and model control groups. However, the protein expression of c-Met after 21 days of TPH treatment was not significantly different from the TP-treated and model control groups.
HGF = hepatocyte growth factor, ELISA = enzyme-linked immunosorbent assay, TPH = attenuated Salmonella typhimurium strain carrying a eukaryotic expression vector encodes the human HGF gene, TP = attenuated Salmonella typhimurium strain with a eukaryotic expression vector.
*P < 0.01, TPH group vs. TP and control groups.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download