1. Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Suppl. 2011; S1–S6.
2. DeFronzo RA, Hompesch M, Kasichayanula S, Liu X, Hong Y, Pfister M, Morrow LA, Leslie BR, Boulton DW, Ching A, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013; 36:3169–3176.
3. Rave K, Nosek L, Posner J, Heise T, Roggen K, van Hoogdalem EJ. Renal glucose excretion as a function of blood glucose concentration in subjects with type 2 diabetes--results of a hyperglycaemic glucose clamp study. Nephrol Dial Transplant. 2006; 21:2166–2171.
4. Ruhnau B, Faber OK, Borch-Johnsen K, Thorsteinsson B. Renal threshold for glucose in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1997; 36:27–33.
5. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39:S1–S266.
6. Devineni D, Morrow L, Hompesch M, Skee D, Vandebosch A, Murphy J, Ways K, Schwartz S. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012; 14:539–545.
7. Devineni D, Curtin CR, Polidori D, Gutierrez MJ, Murphy J, Rusch S, Rothenberg PL. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013; 53:601–610.
8. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–470.
9. Tsuda A, Ishimura E, Ohno Y, Ichii M, Nakatani S, Machida Y, Mori K, Uchida J, Fukumoto S, Emoto M, et al. Poor glycemic control is a major factor in the overestimation of glomerular filtration rate in diabetic patients. Diabetes Care. 2014; 37:596–603.
10. Polidori D, Sha S, Ghosh A, Plum-Mörschel L, Heise T, Rothenberg P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013; 98:E867–71.
11. Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001; 280:F10–F18.
12. Bays H. From victim to ally: the kidney as an emerging target for the treatment of diabetes mellitus. Curr Med Res Opin. 2009; 25:671–681.
13. Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis. 2009; 53:875–883.
14. Wilding JP. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism. 2014; 63:1228–1237.
15. Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005; 54:3427–3434.
16. Chao EC, Henry RR. SGLT2 inhibition--a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010; 9:551–559.
17. Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Parvanova A, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012; 35:2061–2068.
18. Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009; 52:691–697.
19. Zhao F, Zhang L, Lu J, Guo K, Wu M, Yu H, Zhang M, Bao Y, Chen H, Jia W. The Chronic Kidney Disease Epidemiology Collaboration equation improves the detection of hyperfiltration in Chinese diabetic patients. Int J Clin Exp Med. 2015; 8:22084–22097.
20. Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014; 129:587–597.
21. Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes. 2012; 5:313–327.
22. Persson P, Hansell P, Palm F. Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf). 2010; 200:3–10.
23. Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant. 2012; 27:1708–1714.
24. Wuerzner G, Pruijm M, Maillard M, Bovet P, Renaud C, Burnier M, Bochud M. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. Am J Kidney Dis. 2010; 56:303–312.
25. Chandra A, Biersmith M, Tolouian R. Obesity and kidney protection. J Nephropathol. 2014; 3:91–97.
26. Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002; 324:127–137.
27. Hirai K, Ookawara S, Miyazawa H, Ito K, Ueda Y, Kaku Y, Hoshino T, Yoshida I, Tabei K. Low-density lipoprotein apheresis ameliorates monthly estimated glomerular filtration rate declines in patients with renal cholesterol crystal embolism. J Artif Organs. 2015; 18:72–78.
28. Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014; 16:457–466.
29. Nakamura Y, Nagai Y, Terashima Y, Nishine A, Ishii S, Kato H, Ohta A, Tanaka Y. Better response to the SGLT2 inhibitor dapagliflozin in young adults with type 2 diabetes. Expert Opin Pharmacother. 2015; 16:2553–2559.
 ± s)
± s)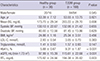
 ± s)
± s)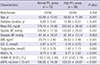






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download