Abstract
Background:
Conventional IgG assays require costly equipment and skilled experts. Semiquantitative measurement of total IgG using point-of-care testing devices may be the solution for these limitations. This study evaluated the reproducibility of the ImmuneCheck™ IgG assay (Proteom-eTech Inc., Korea) and the correlation of its results with conventional laboratory IgG results in the serum and whole blood.
Methods:
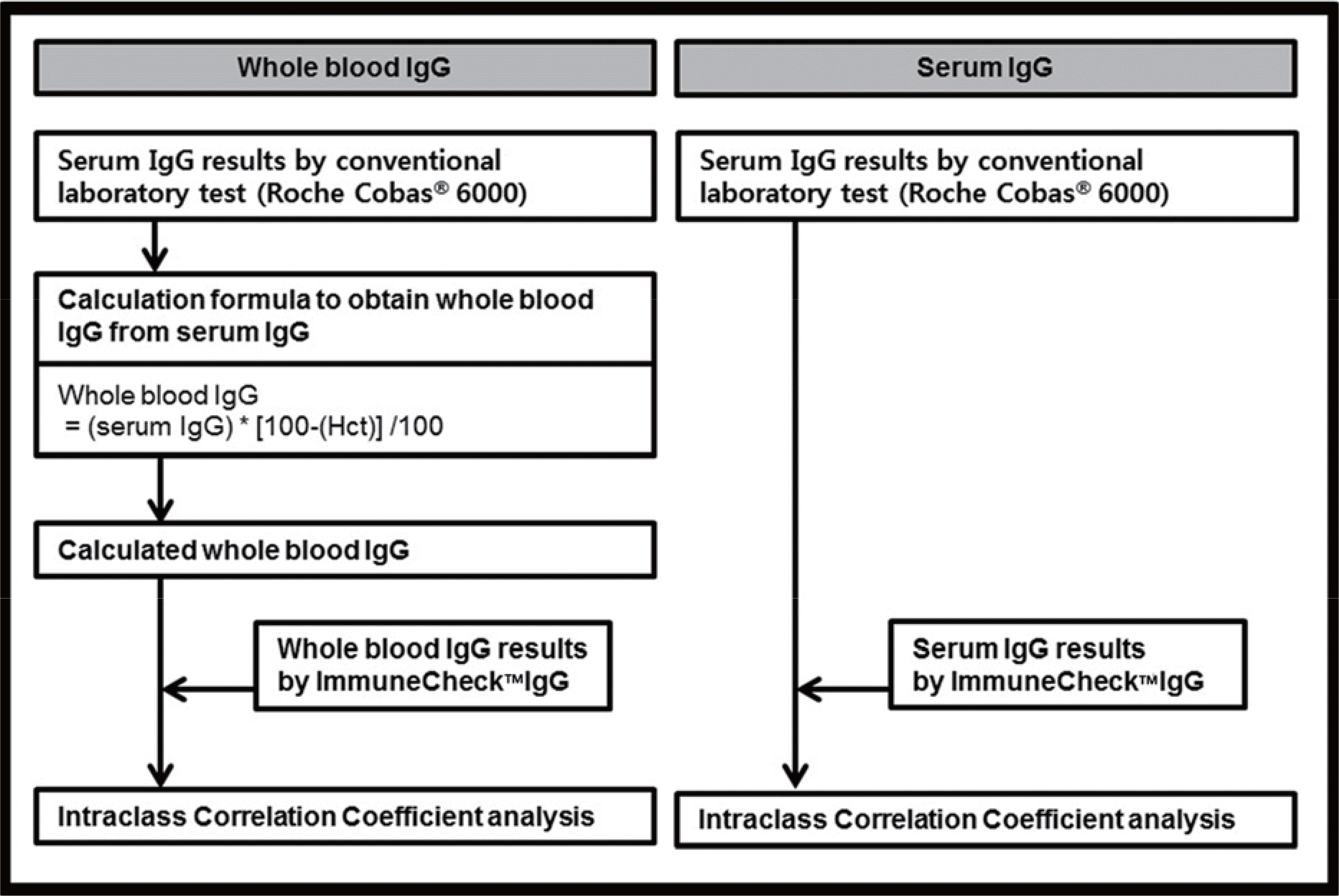
Both the serum and whole blood samples from 120 patients were used. To evaluate the intra-test reproducibility and inter-test correlation, intraclass correlation coefficient (ICC) analysis was used.
Results:
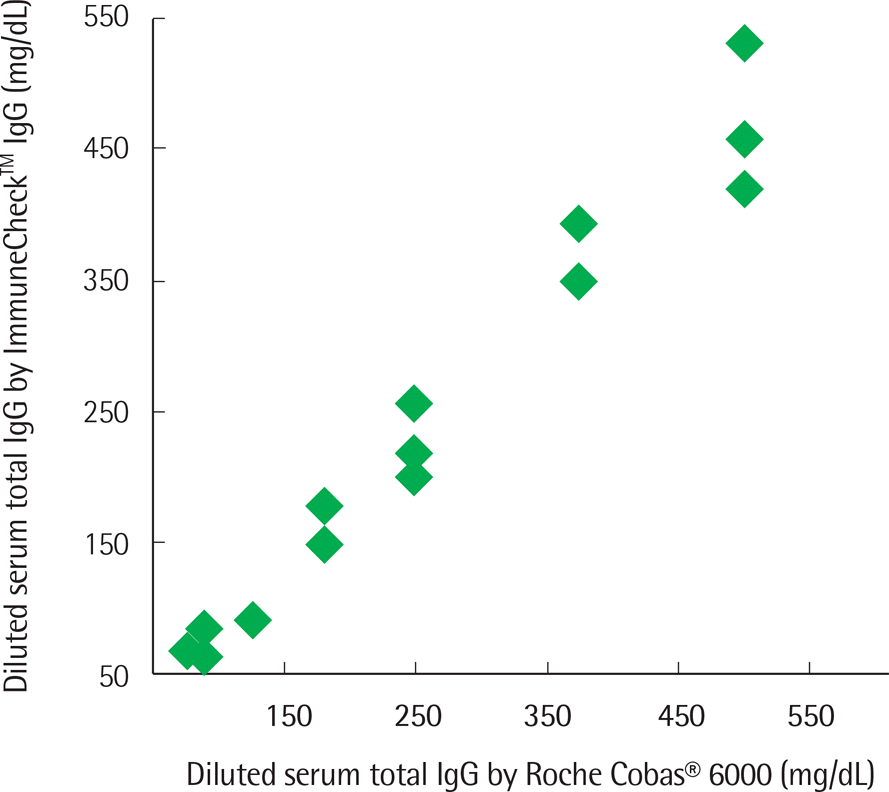
The concentration of serum total IgG measured by cobas® 6000 (Roche Diagnostics, Switzland) ranged from 690.4 to 2,756.4 mg/dL. The intra-test reproducibility of ImmuneCheck™ IgG was high (Serum ICC=0.724, P<0.001; Whole blood ICC=0.843, P<0.001). The inter-test correlation between the ImmuneCheck™ IgG and cobas® 6000 results was very good (Serum ICC=0.805, P<0.001; Whole blood ICC=0.842, P<0.001). Because there were no samples with a total IgG level lower than 600 mg/dL, the pre-existing serum samples were diluted and then the linearity tests were conducted. The intra-test reproducibility for the diluted serum samples was almost perfect (ICC=0.995, P<0.001), and the inter-test correlation between the ImmuneCheck™ IgG and cobas® 6000 results was also strong (ICC=0.992, P<0.001).
Go to : 
REFERENCES
1.Schroeder HW Jr., Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010. 125:S41–52.

2.Aalberse R. The role of IgG antibodies in allergy and immunotherapy. Allergy. 2011. 66(Suppl 95):28–30.

3.Williams JW., Tjota MY., Sperling AI. The contribution of allergen-specifc IgG to the development of th2-mediated airway infammation. J Allergy (Cairo). 2012. 2012:236075.
4.Meulenbroek LA., de Jong RJ., den Hartog Jager CF., Monsuur HN., Wouters D., Nauta AJ, et al. IgG antibodies in food allergy infuence allergen-antibody complex formation and binding to B cells: a role for complement receptors. J Immunol. 2013. 191:3526–33.
5.Carmi Y., Spitzer MH., Linde IL., Burt BM., Prestwood TR., Perlman N, et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature. 2015. 521:99–104.

6.Niu N., Zhang J., Huang T., Sun Y., Chen Z., Yi W, et al. IgG expression in human colorectal cancer and its relationship to cancer cell behaviors. PLoS One. 2012. 7:e47362.

7.Jefferis R., Kumararatne DS. Selective IgG subclass defciency: quan-tifcation and clinical relevance. Clin Exp Immunol. 1990. 81:357–67.
8.Gonzalez-Quintela A., Alende R., Gude F., Campos J., Rey J., Meijide LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008. 151:42–50.

9.Puissant-Lubrano B., Peres M., Apoil PA., Congy-Jolivet N., Roubinet F., Blancher A. Immunoglobulin IgA, IgD, IgG, IgM and IgG subclass reference values in adults. Clin Chem Lab Med. 2015. 53:e359–61.

10.Wood RA., Lederman HM. Immunoglobulin defciency and recurrent upper respiratory tract infections. JAMA. 1987. 257:486.
11.Slatter MA., Gennery AR. Clinical immunology review series: an approach to the patient with recurrent infections in childhood. Clin Exp Immunol. 2008. 152:389–96.

12.Raje N., Dinakar C. Overview of immunodefciency disorders. Immunol Allergy Clin North Am. 2015. 35:599–623.
13.Supak Smolcic V., Bilic-Zulle L., Fisic E. Validation of methods performance for routine biochemistry analytes at Cobas 6000 analyzer series module c501. Biochem Med (Zagreb). 2011. 21:182–90.

14.Landis JR., Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–74.

15.Bonagura VR., Marchlewski R., Cox A., Rosenthal DW. Biologic IgG level in primary immunodefciency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008. 122:210–2.
Go to : 
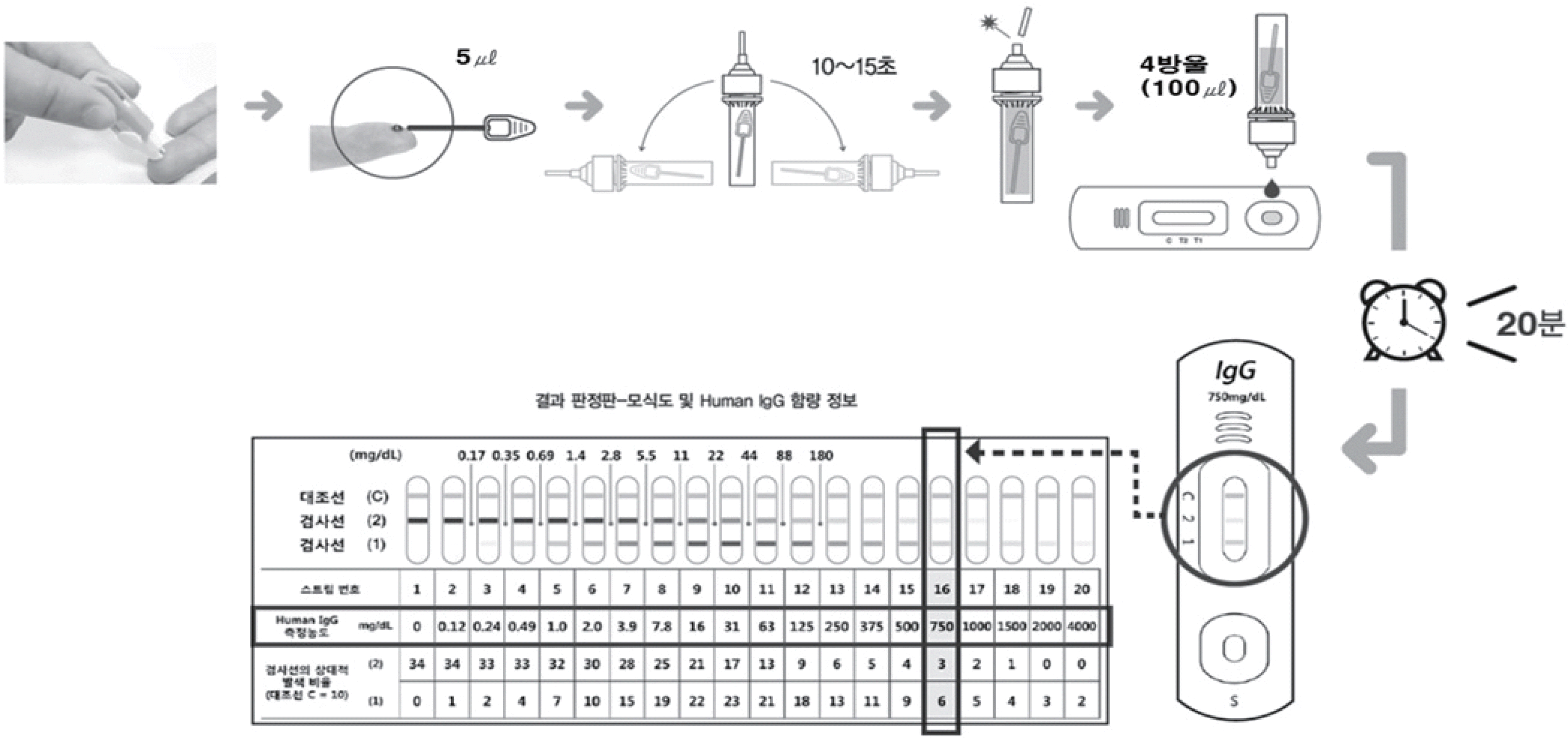
 | Fig. 2.Flowchart of ImmuneCheck™ IgG analysis. Fig. 2. Flowchart of ImmuneCheck™ IgG analysis.
|
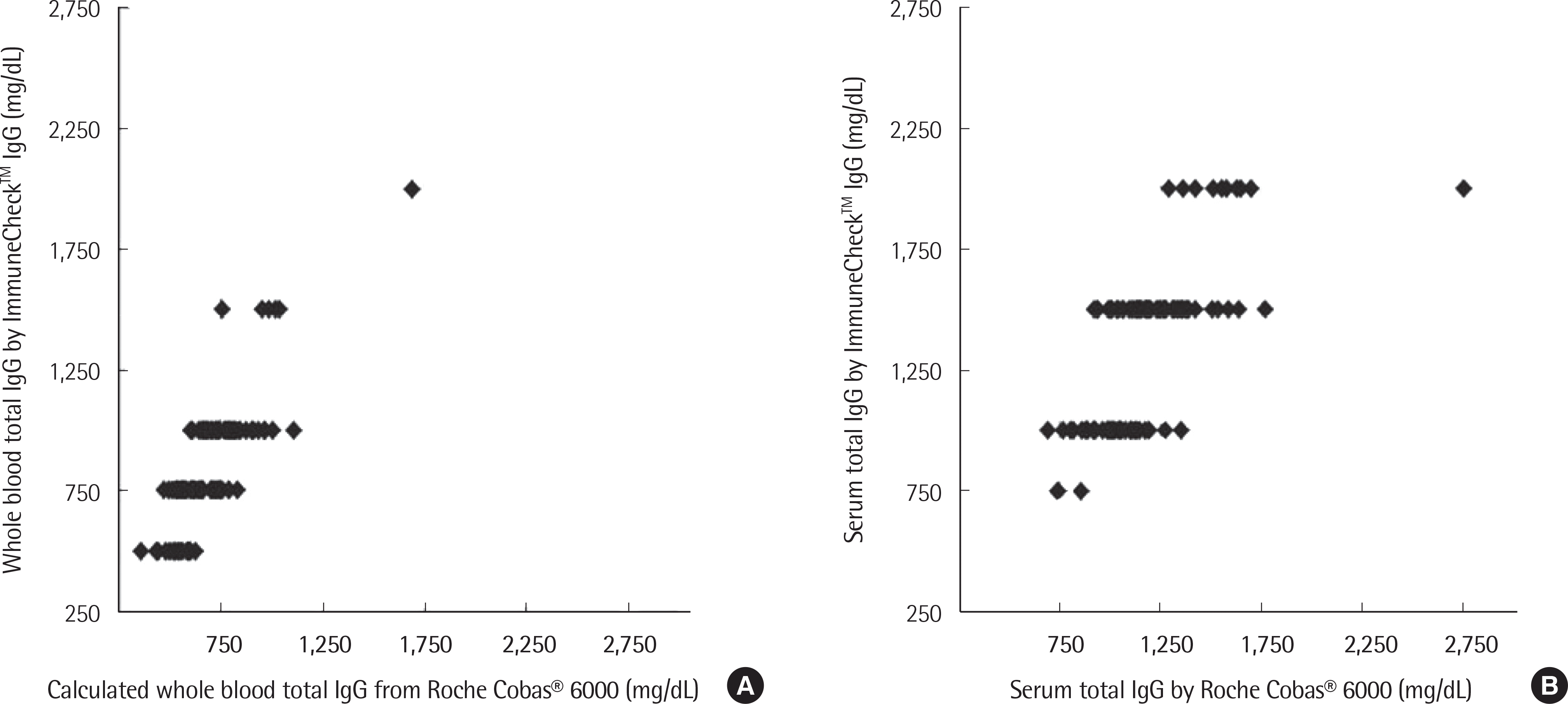 | Fig. 3.Scatter plots of whole blood total IgG titers (A) and serum total IgG titers (B) obtained with ImmuneCheck™ IgG. |
Table 1.
Comparison of baseline characteristics between the male and female patients included in this study
| Item | Males (n=44) | Females (n=76) | P value |
|---|---|---|---|
| Mean age, year (SD) | 41.7 (17.7) | 52.6 (19.4) | 0.003 |
| No. of patients with atopy∗ (%) | 30 (68.2) | 59 (77.6) | 0.258 |
| Mean of hematocrit, % (SD) | 44.9 (3.6) | 39.9 (3.3) | <0.001 |
| Mean of serum total IgG by Roche cobas® 6000, mg/dL (SD) | 1,162.4 (172.8) | 1,196.7 (298.2) | 0.426 |
Table 2.
Comparison of the serum total IgG results obtained with Roche cobas® 6000 and ImmuneCheck™ IgG
Table 3.
ICC analysis of the test results for serum, whole blood, and diluted serum
Table 4.
Inter-test ICC results for serum and whole blood according to age, gender, and hematocrit
Table 5.
Comparison between the conventional laboratory test (Roche cobas® 6000) and ImmuneCheck™ IgG assay




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download