Worldwide,
Campylobacter and nontyphoidal
Salmonella are among the 4 most common causes of foodborne acute diarrheal diseases (
1). Recently,
Campylobacter and nontyphoidal
Salmonella epidemiologic trends have changed in Western countries.
Campylobacter replaced
Salmonella as the most common bacterial pathogen since 1995 in Switzerland (
2). In European Union countries, the prevalence of campylobacteriosis was stationary while that of nontyphoidal salmonellosis (NTS) declined during 2009–2013 (
3).
In Korea, the rates of culture-proven
Campylobacter spp. and nontyphoidal
Salmonella spp. notifications, using stool samples from acute diarrheal patients including adults, were 0.6%–1.4% and 2.1%–2.9%, respectively, according to the 2011–2016 annual reports from the Korea Centers for Disease Control and Prevention (KCDC) sample surveillance system (
4). In a recent Korean study including adults,
Campylobacter was the most frequent bacterial pathogen from diarrheal stools; multiplex polymerase chain reaction (PCR) tests were used for detection (
5).
Few data are available on Campylobacter enterocolitis trends in Korean children. We examined bacterial enteropathogens among hospitalized Korean children with acute inflammatory diarrhea and investigated clinical features of campylobacteriosis vs. NTS, formerly a leading cause of bacterial enterocolitis in Korean children.
This study involved 2 hospitals in southwest Seoul. At hospital A, from 2011 to 2016, fecal samples were collected from hospitalized children aged < 18 years suspected of having acute gastroenteritis as described previously (
67); samples from those with acute inflammatory diarrhea were tested for bacterial pathogens. Acute inflammatory diarrhea was defined as acute diarrhea accompanied by at least one of the following: 1) fever with abdominal cramping, tenesmus, or nighttime diarrhea; 2) leukocyte-positive stool; 3) acute bloody diarrhea or hemoglobin-positive stool; or 4) colonic wall thickening or colitis findings on abdominal sonography or computed tomography. Children with immunosuppressive chronic illnesses, including inflammatory bowel diseases or malnutrition were excluded. Informed consent was obtained from the guardians and/or children. Clinical information was collected including age, sex, hospitalization date and duration, fever duration, duration and maximum number of episodes of diarrhea and vomiting, and medications. Modified Vesikari scores (
8) were calculated and laboratory examinations (complete blood cell count, C-reactive protein level, and leukocyte and hemoglobin stool tests) were conducted.
Storage and processing of fecal samples including DNA extractions were performed as described previously (
67). Multiplex PCR was performed using the Seeplex Diarrhea-B1 and -B2 ACE detection kits (Seegene Inc., Seoul, Korea) according to the manufacturer's instructions to detect
Vibrio spp.,
Clostridium difficile toxin B,
Salmonella spp.,
Shigella spp.,
Campylobacter spp.,
Clostridium perfringens,
Yersinia enterocolitica,
Escherichia coli O157,
E. coli H7, verotoxin-producing
E. coli, and
Aeromonas spp. (
9).
By reviewing medical records from 2014 to 2016, the aforementioned clinical data (except Vesikari scores) and the results of multiplex PCR tests for bacterial pathogens were obtained for children at hospital B with the same inclusion criteria. Multiplex PCR was performed using the same kits at hospital A. Moreover, medical records from 2001 to 2010 of children with stool-culture-proven NTS from the 2 hospitals were reviewed to examine epidemiologic NTS trends. The aforementioned clinical characteristics (except Vesikari scores) were retrospectively analyzed. Statistical analyses were performed using SPSS version 20.0 (IBM Corp., Chicago, IL, USA). The study protocol was approved by the Institutional Review Board of Seoul Metropolitan Government-Seoul National University Boramae Medical Center (IRB No. 02-2011-11, 06-2012-151, 16-2015-78) and Korea University Guro Hospital (KUGH16354). Informed consent was confirmed by the IRB at Boramae Medical Center.
Total 363 children (246 from hospital A, 117 from hospital B) from 2011 to 2016 were included. As the distributions of major bacterial pathogens from the 2 hospitals were mostly similar, combined data are presented (
Fig. 1). Among enteropathogenic bacteria (n = 189, 52.1%; hospitals A and B: n = 130 and 59, 52.8% and 50.4%, respectively),
Campylobacter spp. (n = 68, 18.7%; hospitals A and B: 20.7% and 14.5%, respectively) was most common followed by
Salmonella spp. (n = 56, 15.4%; hospitals A and B: 17.0% and 12.0%) (
Table 1). Of the children, 4.1% were coinfected with
Campylobacter spp. and other bacterial enteropathogen(s); 1.6% were coinfected with
Salmonella spp. and another bacterial enteropathogens. There was no significant seasonal difference between campylobacteriosis and NTS; both were most prevalent during summer (
Table 1).
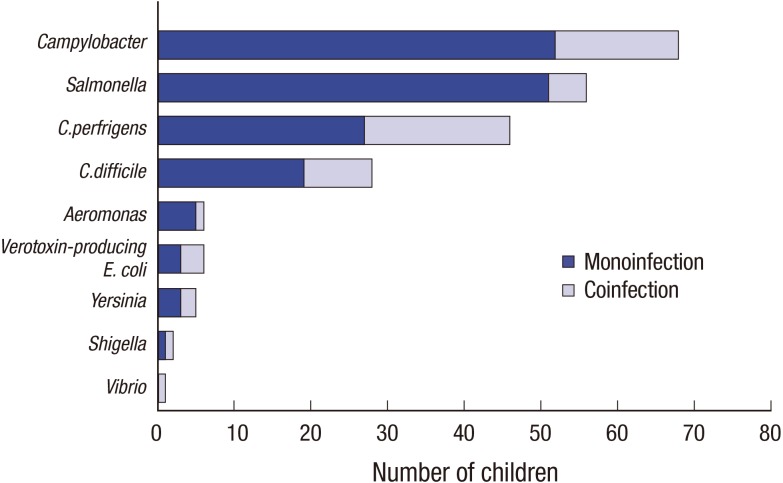 | Fig. 1Prevalence of enteropathogenic bacteria in children with acute inflammatory diarrhea (n = 363). Among enteropathogenic bacteria (n = 189, 52.1%), Campylobacter spp. (n = 68, 18.7%) was most common followed by Salmonella spp. (n = 56, 15.4%). Children coinfected with other bacterial enteropathogens are also shown. 
|
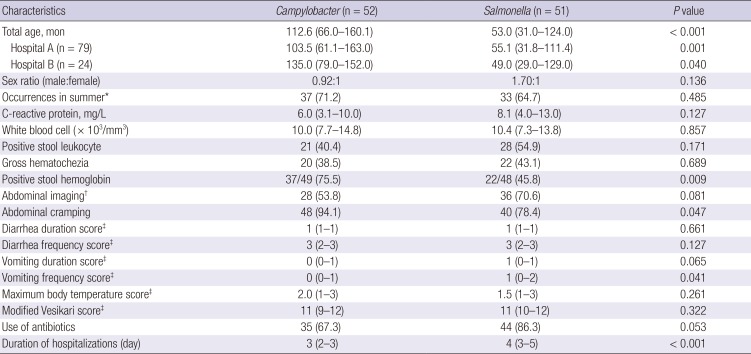
Table 1
Clinical characteristics of children hospitalized with acute gastroenteritis due to Campylobacter or nontyphoidal Salmonella monoinfection (n = 103)
|
Characteristics |
Campylobacter (n = 52) |
Salmonella (n = 51) |
P value |
|
Total age, mon |
112.6 (66.0–160.1) |
53.0 (31.0–124.0) |
< 0.001 |
|
Hospital A (n = 79) |
103.5 (61.1–163.0) |
55.1 (31.8–111.4) |
0.001 |
|
Hospital B (n = 24) |
135.0 (79.0–152.0) |
49.0 (29.0–129.0) |
0.040 |
|
Sex ratio (male:female) |
0.92:1 |
1.70:1 |
0.136 |
|
Occurrences in summer*
|
37 (71.2) |
33 (64.7) |
0.485 |
|
C-reactive protein, mg/L |
6.0 (3.1–10.0) |
8.1 (4.0–13.0) |
0.127 |
|
White blood cell (× 103/mm3) |
10.0 (7.7–14.8) |
10.4 (7.3–13.8) |
0.857 |
|
Positive stool leukocyte |
21 (40.4) |
28 (54.9) |
0.171 |
|
Gross hematochezia |
20 (38.5) |
22 (43.1) |
0.689 |
|
Positive stool hemoglobin |
37/49 (75.5) |
22/48 (45.8) |
0.009 |
|
Abdominal imaging†
|
28 (53.8) |
36 (70.6) |
0.081 |
|
Abdominal cramping |
48 (94.1) |
40 (78.4) |
0.047 |
|
Diarrhea duration score‡
|
1 (1–1) |
1 (1–1) |
0.661 |
|
Diarrhea frequency score‡
|
3 (2–3) |
3 (2–3) |
0.127 |
|
Vomiting duration score‡
|
0 (0–1) |
1 (0–1) |
0.065 |
|
Vomiting frequency score‡
|
0 (0–1) |
1 (0–2) |
0.041 |
|
Maximum body temperature score‡
|
2.0 (1–3) |
1.5 (1–3) |
0.261 |
|
Modified Vesikari score‡
|
11 (9–12) |
11 (10–12) |
0.322 |
|
Use of antibiotics |
35 (67.3) |
44 (86.3) |
0.053 |
|
Duration of hospitalizations (day) |
3 (2–3) |
4 (3–5) |
< 0.001 |

Clinical characteristics were compared between children with campylobacteriosis and those with NTS monoinfection (n = 103). Because the main analysis results were not significantly different between the hospitals, combined data are presented in
Table 1. Children with campylobacteriosis were significantly older than children with NTS; 112.6 months (interquartile range [IQR], 66.0–160.1 months) vs. 53 months (IQR, 31–124 months), respectively (
P < 0.001). Campylobacteriosis and NTS showed significantly different age distributions (
Fig. 2). Relative ratios of campylobacteriosis to NTS were 0, 0.29, 0.59, 1.50, and 2.60 among children aged 6–11 months, 12–23 months, 24–71 months, 6–12 years, and over 13 years, respectively (
P = 0.001). Abdominal cramping (
P = 0.047) and stool hemoglobin (
P = 0.009) were more frequent in children with campylobacteriosis; higher vomiting frequency scores (
P = 0.041) and longer hospitalizations were observed in the NTS group (
P < 0.001).
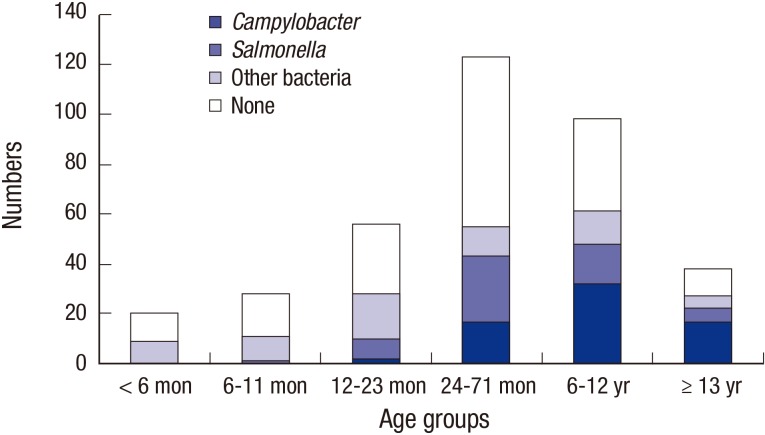 | Fig. 2Enteropathogen notifications in children with inflammatory diarrhea by ages during 2011–2016. An increase in the number of notifications for Campylobacter infection, particularly in older children and school-aged children, is observed (n = 363, P < 0.001). 
|
Because differing age distributions were noted for campylobacteriosis and NTS from 2011 to 2016, ages of children with NTS from 2001 to 2016 (n = 280) were analyzed. Children with stool-culture-proven NTS from 2001 to 2010 were significantly younger than those with NTS or campylobacteriosis from 2011 to 2016 (34.1 months [IQR, 18.7–61.4] vs. 53.0 months or 112.6 months, P < 0.001, respectively); results were similar at both hospitals.
Our study results suggest that campylobacteriosis and NTS may be among the 2 leading causes of acute inflammatory diarrhea in hospitalized Korean children, at least in southwest Seoul. A unique finding is that children with Campylobacter enterocolitis were significantly older than children with NTS; to our knowledge, this is one of the first age-based observations among children. Although it is unclear why this difference in host age exists, it could help to suspect more potential pathogens in children hospitalized with acute inflammatory diarrhea. In younger Korean children, NTS could be suspected as a primary cause; in school-aged children and adolescents, campylobacteriosis could be given priority. This may be helpful for quick decision making regarding initial empirical antimicrobial agents, if necessary.
One assumption regarding this age difference is differing sources of exposure to
Campylobacter and
Salmonella. It was reported that
Campylobacter in broilers had a significant impact on campylobacteriosis in humans with a 2-week lag (
10). In Korean studies, 50%–66% of chicken had
Campylobacter jejuni (
11). Ingestion of contaminated or undercooked chicken may be associated with a higher prevalence of campylobacteriosis in older children, because fast food such as fried chicken may represent the majority of chicken consumption in this age group. Egg products and meat products such as pork were reported to be main sources of NTS (
12). Further epidemiologic studies comparing food consumption patterns of Korean children with campylobacteriosis vs. those with NTS might help explain this age difference. Another explanation could be related to the different minimum amounts of these 2 bacteria causing clinical illness. The minimum infectious dose of
Salmonella spp. was greater than that of
Campylobacter spp. (
13). Thus, under the same exposure conditions, younger children with less gastric acid production may be more prone to salmonellosis than older children. In the literature, NTS was more common in children younger than 5 years than in older children (
141516).
In our study, we noted that the median age of the children with NTS has increased recently, which concurred with the results from a previous Korean study (
14). However, the underlying reasoning remains unclear. Another Korean study reported an association between serotypes and children's ages (
16); however, no significant association between serotypes or serotype changes and age trends was noted in our study, explaining the recent increase observed in the ages of patients with NTS.
The Campylobacter group showed hemoglobin-positive stool and abdominal cramping more frequently than the Salmonella group. However, there was no significant difference in the rates of positivity for leukocytes in stool samples or the need for abdominal imaging. There was a tendency toward higher vomiting severity in NTS, which may suggest greater small bowel involvement. Further large-scale studies are needed to confirm the differences in these clinical findings, considering the relatively low significance noted in a modest number of children. Longer hospitalizations in the Salmonella group might be related to younger age. Younger children need supportive care more frequently and can be dehydrated more frequently, which leads to longer hospitalizations.
In our study, several children with campylobacteriosis or NTS were coinfected with other bacterial enteropathogens; this has been increasingly reported recently (
17). However, it appears that the clinical severity of bacteria-bacteria coinfection (compared with monoinfection) has not been well documented. In our cases, there was no significant difference in clinical severity between monoinfected children and coinfected children.
Because we only included children with acute inflammatory diarrhea, higher notification rates for overall bacterial enteropathogens may have been obtained compared to previous ones (
1819). Higher positive rates for bacteria may be related to use of multiplex PCR tests for detecting bacterial enteropathogens, particularly
Campylobacter spp., as they are more sensitive than culture methods (
9).
In conclusion, there was a high incidence of intestinal campylobacteriosis in hospitalized Korean children. Although the median age of children with NTS increased recently, children with campylobacteriosis were significantly older than those with NTS; they showed more intestinal bleeding and abdominal cramping and less vomiting. We could consider campylobacteriosis rather than NTS as a leading cause of foodborne enterocolitis in hospitalized school-aged Korean children and adolescents.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download