1. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014; 133:621–631. PMID:
24581429.
2. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197. PMID:
26922928.
3. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010; CD001186. PMID:
20687065.
4. Bauer CS, Rank MA. Comparative efficacy and safety of subcutaneous versus sublingual immunotherapy. J Allergy Clin Immunol. 2014; 134:765–765.e2. PMID:
25171871.
5. Nelson HS. Subcutaneous immunotherapy versus sublingual immunotherapy: which is more effective? J Allergy Clin Immunol Pract. 2014; 2:144–149. PMID:
24607040.
6. Casale TB, Stokes JR. Immunotherapy: what lies beyond. J Allergy Clin Immunol. 2014; 133:612–619. PMID:
24581428.
7. Kim ME, Kim JE, Sung JM, Lee JW, Choi GS, Nahm DH. Safety of accelerated schedules of subcutaneous allergen immunotherapy with house dust mite extract in patients with atopic dermatitis. J Korean Med Sci. 2011; 26:1159–1164. PMID:
21935270.
8. Rönmark E, Jönsson E, Lundbäck B. Remission of asthma in the middle aged and elderly: report from the Obstructive Lung Disease in Northern Sweden study. Thorax. 1999; 54:611–613. PMID:
10377206.
9. Panhuysen CI, Vonk JM, Koëter GH, Schouten JP, van Altena R, Bleecker ER, Postma DS. Adult patients may outgrow their asthma: a 25-year follow-up study. Am J Respir Crit Care Med. 1997; 155:1267–1272. PMID:
9105065.
10. Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010; 125:569–574. PMID:
20144472.
11. Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013; 132:1322–1336. PMID:
24139829.
12. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, Cox L, Demoly P, Frew AJ, O’Hehir R, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015; 136:556–568. PMID:
26162571.
13. Tabar AI, Arroabarren E, Echechipía S, García BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. J Allergy Clin Immunol. 2011; 127:57–63. 63.e1–63.e3. PMID:
21211641.
14. Rottem M, Egbarya A. Subcutaneous immunotherapy in Northern Israel: efficacy and safety. Isr Med Assoc J. 2014; 16:539–543. PMID:
25351009.
15. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, Nelson H, Akdis CA. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3. PMID:
23498595.
16. Di Lorenzo G, Mansueto P, Pacor ML, Rizzo M, Castello F, Martinelli N, Ditta V, Lo Bianco C, Leto-Barone MS, D'Alcamo A, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009; 123:1103–1110. 1110.e1–1110.e4. PMID:
19356792.
17. Fujimura T, Yonekura S, Horiguchi S, Taniguchi Y, Saito A, Yasueda H, Inamine A, Nakayama T, Takemori T, Taniguchi M, et al. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin Immunol. 2011; 139:65–74. PMID:
21300571.
18. Ciprandi G, Silvestri M. Serum specific IgE: a biomarker of response to allergen immunotherapy. J Investig Allergol Clin Immunol. 2014; 24:35–39.
19. Tosca M, Silvestri M, Accogli A, Rossi GA, Ciprandi G. Serum-specific IgE and allergen immunotherapy in allergic children. Immunotherapy. 2014; 6:29–33. PMID:
24341881.
20. Passalacqua G. The use of single versus multiple antigens in specific allergen immunotherapy for allergic rhinitis: review of the evidence. Curr Opin Allergy Clin Immunol. 2014; 14:20–24. PMID:
24362238.
21. Webber CM, Calabria CW. Assessing the safety of subcutaneous immunotherapy dose adjustments. Ann Allergy Asthma Immunol. 2010; 105:369–375. PMID:
21055663.
22. Kartal O, Gulec M, Caliskaner Z, Musabak U, Sener O. Safety of subcutaneous immunotherapy with inhalant allergen extracts: a single-center 30-year experience from Turkey. Immunopharmacol Immunotoxicol. 2015; 37:280–286. PMID:
25858053.
23. Santos N, Pereira AM, Silva R, Torres da Costa J, Plácido JL. Characterisation of systemic reactions to subcutaneous immunotherapy with airborne allergens and classification according to WAO 2010. Allergol Immunopathol (Madr). 2015; 43:25–31. PMID:
24661594.
24. Cox L. Advantages and disadvantages of accelerated immunotherapy schedules. J Allergy Clin Immunol. 2008; 122:432–434. PMID:
18619664.
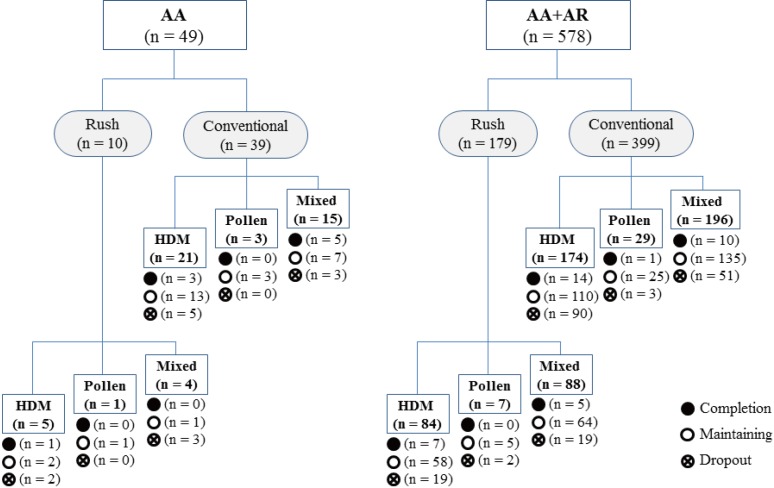
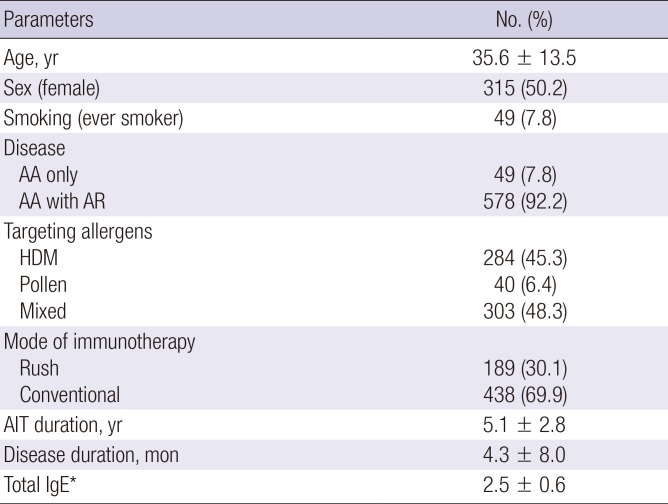
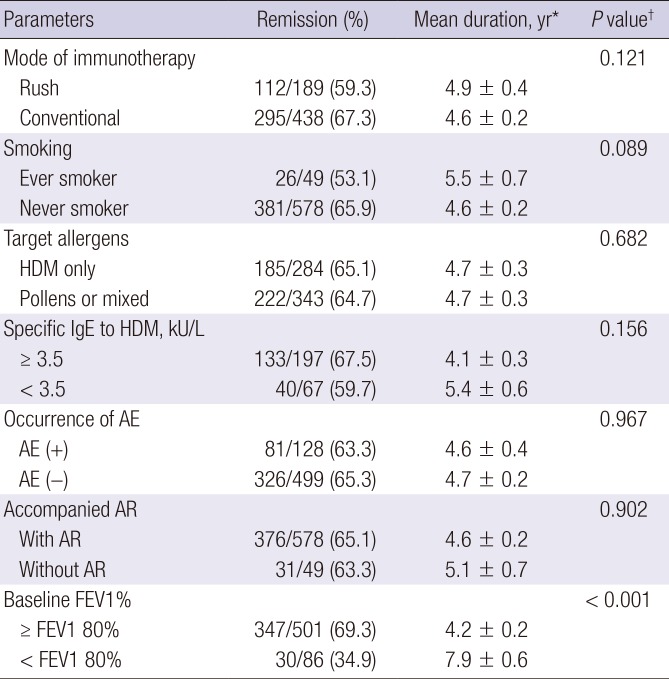
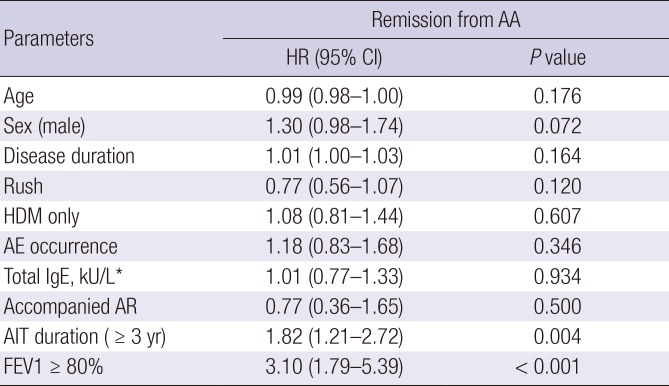




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download