Abstract
Several studies have reported the effect of absorption of procyanidins and their contribution to the small intestine. However, differences between dietary interventions of procyanidins and interventions via antibiotic feeding in pigs are rarely reported. Following 16S rRNA gene Illumina MiSeq sequencing, we observed that both procyanidin administration for 2 months (procyanidin-1 group) and continuous antibiotic feeding for 1 month followed by procyanidin for 1 month (procyanidin-2 group) increased the number of operational taxonomic units, as well as the Chao 1 and ACE indices, compared to those in pigs undergoing antibiotic administration for 2 months (antibiotic group). The genera Fibrobacter and Spirochaete were more abundant in the antibiotic group than in the procyanidin-1 and procyanidin-2 groups. Principal component analysis revealed clear separations among the three groups. Additionally, using the online Molecular Ecological Network Analyses pipeline, three co-occurrence networks were constructed; Lactobacillus was in a co-occurrence relationship with Trichococcus and Desulfovibrio and a co-exclusion relationship with Bacillus and Spharerochaeta. Furthermore, metabolic function analysis by phylogenetic investigation of communities by reconstruction of unobserved states demonstrated modulation of pathways involved in the metabolism of carbohydrates, amino acids, energy, and nucleotides. These data suggest that procyanidin influences the gut microbiota and the intestinal metabolic function to produce beneficial effects on metabolic homeostasis.
Recent studies have indicated that the gut microbiota acts as a major environmental factor and is associated with diabetes [32], Parkinson's disease [37], and metabolic syndrome [43]. The composition of the gut microbiota is influenced by various factors, including host genetics, immunological factors, antibiotic usage [1334], and dietary habits [69]. Diet can significantly affect the gut environment by dominating the host genotype [4], impacting gut transit time [18], etc. In addition, changing the consumption of carbohydrates, proteins, and fats can influence the composition of the microbiota [38]. Thus, dietary habits can markedly influence gut microbiota directly, or through interaction with dietary components, and may serve as a new target in the study of preventing and treating diseases.
Pine bark has been utilized throughout the world as a nutritional supplement and a phytochemical remedy for various diseases [36]. Pine bark extracts have received considerable attention due to their pharmacological effects, in particular on anti-colorectal cancer [20], anti-inflammatory [15], anti-atherosclerosis [19], and anti-chronic ultra violet B radiation activity [21]. Procyanidins, which are present in extracts of Pinus koraiensis bark, have antioxidant activity in DPPH stable radicals [17], antitumor activity in mice bearing U14 cervical cancer [24], and can decrease activity against Staphylococcus aureus [30]. Although the antibacterial activity of procyanidins is moderate compared with that of antibiotics, the real antibacterial benefit of procyanidins may be that they inhibit β-lactamase, thus decreasing the stability of the bacterial cell membrane and the minimum inhibitory concentrations of antibiotics for drug-resistant bacteria like S. aureus [22]. In addition, procyanidins have been proposed as preventive agents owing to their antiproliferative effects [1127]. Ingestion of procyanidins has been shown to enhance absorption of the small intestine [41] and hamper obesity associated with gut microbial and metabolomic changes [28].
The pig is becoming an important animal model due to its physiological and anatomical similarity to humans. Compared with domestic pigs, miniature pigs (minipigs) have the advantages of small body size, ease of handling, a diet similar to humans, ease of standardized quality control, and less test substrate required for biomedical applications. Bama minipigs, a distinct breed of miniature swine present in China, are, in many regards, ideal animal models of humans [2326] and are considered suitable medical laboratory animals due to their genetic stability, high inbred status, small body, and high resistance to disease [39]. Consequently, the assessment of whether or not the ingestion of dietary procyanidins in Bama minipigs actually results in changes to the gut microbiota would not only provide theoretical insight into potential procyanidin-related pharmacologic effects but would also enable a more significative interpretation of procyanidin consumption and decreased disease risk.
Previous studies have reported the absorption of procyanidins as well as their contribution to the small intestine [116]. However, studies comparing well-controlled dietary interventions of procyanidins with antibiotic feeding in pigs are rarely reported. Although earlier studies showed the stability of procyanidins in the stomach [35], it remains unclear whether procyanidins could replace antibiotics in feeding and, more importantly, whether they could enhance intestinal bacterial metabolic function. In the current study, we fed pigs a conventionally designed diet that provided procyanidin, either in combination with antibiotics or individually, to compare the role of procyanidin with antibiotics in gut microbiota.
All experiments involving female Bama minipigs were approved by the Animal Ethical Committee of Shanghai Jiao Tong University prior to beginning the experiment (animal ethics approval No. 201040). Sixty-three Bama minipigs, aged 2 months and weighing 10 ± 1.5 kg, were divided into three groups averagely, the antibiotic group (fed a conventional feed containing 350 mg/kg chlortetracycline and 36 mg/kg tiamulin), procyanidin-1 group (feed included procyanidin at 250 mg/kg body weight), and the procyanidin-2 group (fed conventional antibiotic feed followed by procyanidin-supplemented feed) and were housed in three separate pens in the same compartment. The diets were formulated to meet the animal's nutrient requirements and differed among each other in antibiotic and procyanidin levels. Bama minipigs in the antibiotic group received antibiotic-containing feed for two months, pigs in the procyanidin-1 group received procyanidin-supplemented feed for 2 months, and pigs in the procyanidin-2 group received antibiotic-containing feed for 1 month followed by procyanidin-supplemented feed for 1 month.
Fecal samples (3–5 g) were collected using a sterile cotton swab at the end of two months. Thirteen fecal samples from the antibiotic group (No. A101–A113), 18 fecal samples from the procyanidin-1 group (No. P101–P118) and 21 fecal samples from the procyanidin-2 group (No. P201–P221) were selected for investigation of the adaptation of the gut microbiota to the experimental diets. Samples were collected on ice and immediately stored at −80℃ until subsequent analysis.
Total DNA of the microorganisms obtained from the fecal swabs was extracted using the Tiangen stool mini kit (TianGen, China). The extracted DNA was distinguished by performing 0.8% agarose gel electrophoresis and quantified by an ultra violet spectrophotometer. The V3-V4 region of 16S rRNA was amplified with primer pair 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The oligonucleotide sequence ‘barcode’ was added to differentiate samples from within the same library. PCR was performed with the PCR products purified from 2% agarose gel electrophoresis by using an AxyPrep DNA Gel Extraction kit (AxyGen, USA).
A Quant-iT PicoGreen dsDNA Assay Kit and a microplate reader (FLx800; BioTek, USA) were used to quantify the PCR products. A single-indexed Illumina library was constructed with the TruSeq Nano DNA LT Sample Prep Kit (Illumina, USA). To improve the quality of the Miseq sequencing data, defective reads, including readers without recognizable reverse primer, shorter than 150 bp, and containing ambiguous bases, were removed after trimming the barcodes. The optimal insertion fragment ranged from 200 to 450 bp.
Sequences with a 97% similarity cutoff were clustered into one operational taxonomic unit (OTU) by using the Ribosomal Database Project (RDP) rRNA Classifier. Quality-filtering of de-multiplexed reads was performed by removing low-abundance OTUs, and data were analyzed using QIIME 1.8.0 [3]. The GreenGenes database release 13.8 (Second Genome, USA) was used for open reference OTU picking and taxonomy assignment, and bacterial sequences were aligned by using UCLUST. Species classification annotations were conducted using Silva database release 115 [33], and species-classified information was obtained. The composition of each sample was calculated at the classification levels of phylum, class, order, family, genus, and species. Based on the OTUs, indicators of alpha diversity contained Shannon, species richness estimator of Chao1, ACE, and Simpson indices were generated by using QIIME V1.8.0 [3]. Linear discriminant analysis of the effect size was performed to estimate taxonomic abundance and characterize differences among the groups. The UniFrac distance between categorized samples was visualized by using Cytoscape software (v2.8.3) [40].
The correlation coefficients of Spearman correlation matrix among the top 50 abundant bacterial genera were calculated, and a co-occurrence network was constructed using the online Molecular Ecological Network Analyses (MENA) pipeline, which implements rho > 0.6 and p < 0.01 values for threshold identification. In the co-occurrence networks, OTUs were represented by network nodes and correlations were transformed into links between them. Co-occurrence networks were then visualized by using Cytoscape.
Functional gene prediction was performed using phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) 1.0.0 and GreenGenes database v13.5 (Second Genome). By removing copies of the 16S marker gene in the genomics of species, PICRUSt could normalize the abundance of OTUs, calculate the abundance of corresponding clusters of orthologous groups of proteins, predict metagenomic functional content and Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology abundance from the GreenGenes-picked OTUs. Base on the relative abundance of bacteria at the genus level, Venn diagrams were generated to visualize the shared and unique OTUs among samples or groups by using the R package “VennDiagram”, based on the occurrence of OTUs across samples/groups regardless of their relative abundance [50].
Differences in the UniFrac distances for pairwise comparisons among groups were determined using Student's t-test. The significance of differentiation of microbiota structure among groups was assessed by permutational multivariate analysis of variance [29] and analysis of similarities [47] using R package “vegan”. Taxa abundances at the phylum, class, order, family, genus, and species levels were statistically compared among samples or groups by using Metastats. Linear discriminant analysis effect size was performed to detect differentially abundant taxa across groups using the default parameters. Partial least squares discriminant analysis was introduced as a supervising model to reveal microbiota variation among groups, using the “plsda” function in the R package “mixOmics”. Generalization error was estimated using 10-fold cross-validation. The expected “baseline” error was also included, which was obtained by a classifier that simply predicts the most common category label. Co-occurrence analysis was performed by calculating Spearman's rank correlations between predominant taxa.
Totals of 2,628, 3,118, and 3,620 OTUs were obtained in the antibiotic, procyanidin-1, and procyanidin-2 groups, with 1,686 common OTUs in the three groups. The classifications of the relative abundances of intestinal tract OTUs from phylum, class, order, family, genus, and species are depicted in Fig. 1. On the basis of the classifications (> 1%), the number of OTUs at phylum, order, family and genus levels in the antibiotic group were lower than that in the procyanidin-1 and procyanidin-2 groups (p < 0.05; Table 1). No significant differences in the number of OTUs at different classification levels were found between the procyanidin-1 and procyanidin-2 groups.
Rarefaction curves are indicative of abundant cumulative species richness which serves as a function of the numbers of individuals sampled. Rank-abundance curves can reflect abundance through the length of the curve on the horizontal axis and indicate evenness of species by the shape of the curve. Analysis of these data revealed that the rarefaction curve was smooth (panel A in Fig. 2), indicating that the sample size is sufficient to reflect the richness of the community. Additionally, the smoothness of the rank-abundance curve suggested the presence of high abundance and evenness among samples (panel B in Fig. 2).
To analyze the α diversity in the gut microbiota of Bama minipigs, the OTU richness (Chao1), Shannon, ACE, and Simpson indices were calculated (Fig. 3). No significant differences were observed in either Shannon (panel A in Fig. 3) or Simpson (panel D in Fig. 3) indices between the antibiotic group and the procyanidin-1 or procyanidin-2 groups. However, the Chao 1 (panel B in Fig. 3) and ACE (panel C in Fig. 3) indices in the antibiotic group were significantly lower than those in the procyanidin-1 and procyanidin-2 groups (p < 0.05). No significant differences in the α diversity index were found between the procyanidin-1 and procyanidin-2 groups. Taken together, these results suggest that the diversity of Bama minipigs gut microbiota was not significantly affected by antibiotics, but microbiota richness was significantly reduced with the use of antibiotics.
The composition of the gut microbiota communities at the phylum level is shown in Fig. 4. Among the phyla, Firmicutes were the most predominant. The proportions of Firmicutes in the procyanidin-1 (58.22%) and procyanidin-2 (67.98%) groups were higher than that in the antibiotic group (58.62%, p < 0.01). However, no differences in the relative ratio of Firmicutes to Bacteroidetes were found between the antibiotic group and the procyanidin-1 or procyanidin-2 groups. Differences in the gut microbiota between antibiotic and procyanidin-fed Bama minipigs were also examined. In the antibiotic group, Bacteroidales was the most abundant, while in the procyanidin-1 group, Coprococcus was the most abundant, and in the procyanidin-2 group, Bacilli was the most abundant (panel A in Fig. 5). In addition, differences in the gut microbiota among the groups are also visualized by reviewing the classification tree (panel B in Fig. 5). In the antibiotic group, the abundances of the genera Spirochaetes and Fibrobacteres were significantly different from those in the procyanidin-1 and procyanidin-2 groups. The genus Coprococcus was more abundant in the procyanidin-1 group than in the antibiotic and procyanidin-2 groups. The genera Megasphaera, Blautia, and Lactobacillale were more abundant in the procyanidin-2 group than in the other two groups. The genera Fibrobacter and Spirochaete were more abundant in the antibiotic group than in the two procyanidin groups.
PCoA analysis was performed based on unweighted UniFrac and Bray-Curtis distance matrices of the 16S rRNA sequences. As shown in Fig. 6, the sample contribution rates of the first PCoA (PC1), second PCoA (PC2), and third PCoA (PC3) were 11.85%, 4.09%, and 2.84%, respectively, indicating a clear separation of the antibiotic, procyanidin-1, and procyanidin-2 groups.
In addition, we employed co-occurrence and co-exclusion analysis between genus-level clades throughout the microbiome based on the significant Spearman's rank correlation values (rho > 0.6, p < 0.01; Fig. 7). Notably, the genus Turicibacter had the most nodes and formed co-occurrence relationships with Adlercreutzia, Anaerovorax, Trichococcus, Desulfovibrio, and Lactobacillus, and only formed a co-exclusion relationship with Faecalibacterium. Additionally, Lactobacillus formed co-occurrence relationships with Trichococcus and Desulfovibrio and had co-exclusion relationships with Bacillus and Spharerochaeta.
Based on the results of closed-reference OTU picking, the functional content of the gut microbiota was inferred by using PICRUSt. At an FDR of 0.05, the relative abundances of the KEGG pathways at level 2 encoded in the microbiota showed that carbohydrate metabolism, amino acid metabolism, energy metabolism, and nucleotide metabolism were the predominant microbiotal activities (panel A in Fig. 8). Compared to the antibiotic group, an increase of the pathway involved in carbohydrate metabolism and a decrease in the pathway involved in amino acid metabolism were detected in the procyanidin-1 and procyanidin-2 groups. Of the functional groups detected in the fecal samples, 5,052 were detected in all three groups, with 5,180 functional groups in the antibiotic group, 5,612 functional groups in the procyanidin-1 group, and 5,614 functional groups in the procyanidin-2 group (panel B in Fig. 8).
Gut microflora improvement in pigs is beneficial to animal translational research, husbandry optimization, and animal health improvement. Frese et al. [14] described dietary shaped gut microbiomes of young pigs during nursing and weaning. Similarly, Tilocca et al. [42] reported a diet-dependent shift of the pigs' fecal microbiota composition, emphasizing the importance of diet as a factor shaping the structural and functional composition of pigs' intestinal microbiota. In the current study, antibiotic- or procyanidin-supplemented diets were fed to Bama minipigs for 2 months or antibiotics were fed for 1 month followed by procyanidin for 1 month in order to elucidate the effect of procyanidin on minipigs' gut microbiota. We observed that procyanidin feeding produced a distinct fecal microbiome in Bama minipigs, resulting in increased gut microbiota richness, and decreased or increased abundance compared with feeding antibiotic. Our data indicate the potential of ingesting of dietary procyanidin as a replacement for dietary antibiotics in pig breeding.
An increase in bacterial α-diversity is associated with better health [7], and this has been shown in both animal models and clinical studies [225]. In our study, analysis of α-diversity showed no significant differences in either Shannon or Simpson indices between the antibiotic group and procyanidin groups; however, the antibiotic group showed decreased species richness compared with that in the procyanidin group. Decreased species richness in the antibiotic group hints at the importance of gut microbiota in disease exacerbation. Future research into the composition of gut microbiota communities might help elucidate the role of procyanidins in gut microbiota. We then observed that the proportions of the Firmicutes phylum in the procyanidin-1 and procyanidin-2 groups were higher than that in the antibiotic group. A study in adults with coronary artery disease showed an increase in the level of phylum Firmicutes in patients [10]. Additionally, bacteria from the phylum Firmicutes has been associated with obesity [44] and antibiotic treatment [5]. Thus, factors associated with an increase in Firmicutes in the procyanidin-1 and procyanidin-2 groups may be multiple.
The genera Fibrobacter and Spirochaete were more abundant in the antibiotic group than in the procyanidin-1 and procyanidin-2 groups. Fibrobacter was shown to be significantly affected by Trichuris suis infection in porcine colon microbiota [48]. Spirochaetes are prominent in polymicrobial infections that cause periodontal diseases in humans and equines [846]. Future studies on a large pig cohort and additional metagenomic research may be needed to help define the role of procyanidins in gut microbiota.
Microorganisms rarely survive in isolation, instead, they typically coexist in complex ecological situations involving various symbiotic relationships [12]. In contrast to previous pig gut bacteria reports, co-exclusion of Turicibacter and Lactobacillus was observed in boar Duroc, Yorkshire, Landrace, and Hampshire pigs [49], whereas we detected co-occurrence of Turicibacter and Lactobacillus. Groups of densely connected molecules commonly share vital biological significances in interaction networks [31]. Observing co-occurring genera and co-excluding genera in the current study may indicate some fundamentally important functions and such relationships need to be studied further.
Functional analysis using PICRUSt showed modulation of pathways involved in the metabolism of carbohydrates, amino acids, energy, and nucleotides, which is consistent with our observations based on abundance. We observed an increase in the pathway involved in carbohydrate metabolism and a decrease in the pathway involved in the amino acid metabolism in the procyanidin-1 and procyanidin-2 groups compared to the antibiotic group. However, in the current study, the application of the high-throughput sequencing method alone does have limitations, as it does not provide direct data on the functionally important changes in microbiota. Large research studies that include time series of samples and multiple methodologies to study the functional changes in gut microbiota are needed to evaluate the specific role of procyanidin in gut microbiota.
In a previous randomized, controlled, double-blind, crossover intervention study, researchers reported that daily consumption of a high level of cocoa flavanol for 4 weeks could significantly increase the levels of bifidobacteria and lactobacilli, and decrease clostridia levels, suggesting a potential effect of cocoa procyanidins on gut microflora in humans [45]. In this study, the procyanidin-2 group received antibiotic-supplemented feed for 1 month and procyanidin-supplemented feed for the following month. Although PCoA analysis separated the procyanidin-1 and procyanidin-2 groups clearly, there were no significant differences in the number of OTUs at different classifications nor in the α-diversity indices between the two groups, suggesting that feeding procyanidin for 1 month can change the gut microbiota of Bama minipigs.
By combining the results on the number of OTUs at different classifications, the α-diversity index, and the characterization and functional analysis of gut microbiota, the current study preliminarily illustrates the different features of Bama minipigs gut microbiota under antibiotic feeding, procyanidin feeding, and antibiotic + procyanidin feeding conditions. In addition, our results provide evidence that dietary procyanidins have beneficial effects on the intestinal flora of pigs by improving the richness and abundance of intestinal flora and enhancing metabolic functions, suggesting that dietary procyanidins could act as a substitute for antibiotics in pig feeding programs. Nevertheless, the obtained functional profiles are merely a prediction and, to better elucidate the treatment effects of procyanidins and antibiotics, further studies that include biological controls are needed to determine the contributions of the gut microbiota to the health and physiology of Bama minipigs.
Figures and Tables
 | Fig. 1Operational taxonomic unit (OTU) classification levels and relative abundance of intestinal tract OTUs. The horizontal coordinate is arranged according to the sample name, and the ordinate is the number of OTUs that are classified into phylum, class, order, family, genus and species levels for each sample from the three study groups. |
 | Fig. 2Rarefaction curves (A) and rank-abundance curves (B) at 97% similarity levels. The number of operational taxonomic units (OTUs) acts as a function of the number of sequence tags sampled. |
 | Fig. 3The α diversity index in the antibiotic group compared with those in the procyanidin groups. (A) Shannon index, (B) Chao1, (C) ACE, and (D) Simpson index. A, antibiotic group; P1, procyanidin-1 group; P2, procyanidin-2 group. *p < 0.05 compared with the antibiotic group. |
 | Fig. 4Relative abundance of operational taxonomic units at the phylum level. Each color indicates one phylum. For each color, the height of the column indicates the abundance of reads. |
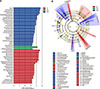 | Fig. 5Microbial differences in the bacterial community among groups categorized according to antibiotic and procyanidin treatment. (A) Bacterial classification between the antibiotic (A), procyanidin-1 (P1), and procyanidin-2 (P2) groups of Bama minipigs; (B) Cladogram from the linear discriminant analysis (LDA) of the effect size (LEfSe) results in the three study groups. |
 | Fig. 6The principal component analysis (PCoA) analysis of the gut microbiota samples. Unweighted Unifrac separates the antibiotic (red), procyanidin-1 (blue), and procyanidin-2 (yellow) groups. |
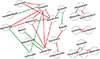 | Fig. 7Network of the co-occurring 90% cutoff operational taxonomic units based on correlation analysis. A connection indicates strong (Spearman's rho > 0.6) and significant (p < 0.01) correlations. The connection between nodes indicates that there is a correlation between the two species (red, positive correlation; green, negative correlation). The size of each node is proportional to the degree of connectivity. |
 | Fig. 8Prediction of bacterial metabolic function. (A) Comparison of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways predicted using phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) according to antibiotic or procyanidin treatment. (B) Venn diagram of the common functional groups. A, antibiotic group; P1, procyanidin-1 group; P2, procyanidin-2 group. |
Acknowledgments
This work was financially supported by The National Key Research and Development Program of China (2017YFC1200202) and the Shanghai Agriculture Applied Technology Development Program, China (grant No. HuNongKe (2017) 1-11).
References
1. Appeldoorn MM, Vincken JP, Gruppen H, Hollman PC. Procyanidin dimers A1, A2, and B2 are absorbed without conjugation or methylation from the small intestine of rats. J Nutr. 2009; 139:1469–1473.

2. Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, Leung JM, Wiens KE, Vujkovic-Cvijin I, Kim CC, Yarovinsky F, Lerche NW, McCune JM, Loke P. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog. 2012; 8:e1003000.

3. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; 7:335–336.

4. Carmody RN, Gerber GK, Luevano JM Jr, Gatti DM, Somes L, Svenson KL, Turnbaugh PJ. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015; 17:72–84.

5. Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012; 488:621–626.

6. Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, Fitzgerald GF, Deane J, O'Connor M, Harnedy N, O'Connor K, O'Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O'Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012; 488:178–184.

7. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012; 148:1258–1270.

8. Cox A, Dixon P, Smith S. Histopathological lesions associated with equine periodontal disease. Vet J. 2012; 194:386–391.

9. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010; 107:14691–14696.

10. Emoto T, Yamashita T, Sasaki N, Hirota Y, Hayashi T, So A, Kasahara K, Yodoi K, Matsumoto T, Mizoguchi T, Ogawa W, Hirata K. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016; 23:908–921.

11. Faria A, Calhau C, de Freitas V, Mateus N. Procyanidins as antioxidants and tumor cell growth modulators. J Agric Food Chem. 2006; 54:2392–2397.

12. Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, Huttenhower C. Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol. 2012; 8:e1002606.

13. Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012; 3:289–306.

14. Frese SA, Parker K, Calvert CC, Mills DA. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015; 3:28.

15. Gonçalves C, Dinis T, Batista MT. Antioxidant properties of proanthocyanidins of Uncaria tomentosa bark decoction: a mechanism for anti-inflammatory activity. Phytochemistry. 2005; 66:89–98.

16. Gulgun M, Erdem O, Oztas E, Kesik V, Balamtekin N, Vurucu S, Kul M, Kismet E, Koseoglu V. Proanthocyanidin prevents methotrexate-induced intestinal damage and oxidative stress. Exp Toxicol Pathol. 2010; 62:109–115.

17. Jerez M, Deive FJ, Sineiro J, Núñez MJ. Antioxidant activity of pine bark procyanidins in bulk corn oil and corn oil-in-water emulsions. Eur J Lipid Sci Tech. 2011; 113:1402–1411.

18. Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M, Tache Y, Pasricha PJ, Knight R, Farrugia G, Sonnenburg JL. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013; 144:967–977.

19. Kim DS, Kim MS, Kang SW, Sung HY, Kang YH. Pine bark extract enzogenol attenuated tumor necrosis factor-α-induced endothelial cell adhesion and monocyte transmigration. J Agric Food Chem. 2010; 58:7088–7095.

20. Kim YJ, Park HJ, Yoon SH, Kim MJ, Leem KH, Chung JH, Kim HK. Anticancer effects of oligomeric proanthocyanidins on human colorectal cancer cell line, SNU-C4. World J Gastroenterol. 2005; 11:4674–4678.

21. Kimura Y, Sumiyoshi M. French maritime pine bark (Pinus maritima Lam.) extract (Flavangenol®) prevents chronic UVB radiation-induced skin damage and carcinogenesis in melanin-possessing hairless mice. Photochem Photobiol. 2010; 86:955–963.

22. Kusuda M, Inada K, Ogawa TO, Yoshida T, Shiota S, Tsuchiya T, Hatano T. Polyphenolic constituent structures of Zanthoxylum piperitum fruit and the antibacterial effects of its polymeric procyanidin on methicillin-resistant Staphylococcus aureus. Biosci Biotechnol Biochem. 2006; 70:1423–1431.

23. Li J, Liu Y, Zhang JW, Wei H, Yang L. Characterization of hepatic drug-metabolizing activities of Bama miniature pigs (Sus scrofa domestica): comparison with human enzyme analogs. Comp Med. 2006; 56:286–290.
24. Li K, Li Q, Li J, Zhang T, Han Z, Gao D, Zheng F. Antitumor activity of the procyanidins from Pinus koraiensis Bark on mice bearing U14 cervical cancer. Yakugaku Zasshi. 2007; 127:1145–1151.

25. Lin A, Bik EM, Costello EK, Dethlefsen L, Haque R, Relman DA, Singh U. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS One. 2013; 8:e53838.

26. Liu Y, Zeng BH, Shang HT, Cen YY, Wei H. Bama miniature pigs (Sus scrofa domestica) as a model for drug evaluation for humans: comparison of in vitro metabolism and in vivo pharmacokinetics of lovastatin. Comp Med. 2008; 58:580–587.
27. Mantena SK, Baliga MS, Katiyar SK. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis. 2006; 27:1682–1691.

28. Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep. 2016; 6:31208.

29. McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 2001; 82:290–297.

30. Ming DS, López A, Hillhouse BJ, French CJ, Hudson JB, Towers GH. Bioactive constituents from Iryanthera megistophylla. J Nat Prod. 2002; 65:1412–1416.
31. Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci U S A. 2006; 103:8577–8582.

32. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012; 490:55–60.

33. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013; 41:D590–D596.

34. Relman DA. Microbiology: learning about who we are. Nature. 2012; 486:194–195.
35. Rios LY, Bennett RN, Lazarus SA, Rémésy C, Scalbert A, Williamson G. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr. 2002; 76:1106–1110.

36. Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002; 40:158–168.

37. Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015; 30:350–358.

38. Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013; 69:52–60.

39. Shang HT, Niu R, Wei H, Huang ZB, Gan S, Wang A, Zeng Y. Genetic analysis of 35 microsatellite loci in three miniature pig breeds. Hereditas. 2001; 23:17–20.
40. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13:2498–2504.

41. Spencer JP, Schroeter H, Rechner AR, Rice-Evans C. Bioavailability of flavan-3-ols and procyanidins: gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxid Redox Signal. 2001; 3:1023–1039.

42. Tilocca B, Burbach K, Heyer CME, Hoelzle LE, Mosenthin R, Stefanski V, Camarinha-Silva A, Seifert J. Dietary changes in nutritional studies shape the structural and functional composition of the pigs' fecal microbiome–from days to weeks. Microbiome. 2017; 5:144.

43. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012; 489:242–249.

44. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009; 457:480–484.

45. Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr. 2011; 93:62–72.

46. Visser MB, Ellen RP. New insights into the emerging role of oral spirochaetes in periodontal disease. Clin Microbiol Infect. 2011; 17:502–512.

47. Warton DI, Wright ST, Wang Y. Distance-based multivariate analyses confound location and dispersion effects. Method Ecol Evol. 2012; 3:89–101.

48. Wu S, Li RW, Li W, Beshah E, Dawson HD, Urban JF Jr. Worm burden-dependent disruption of the porcine colon microbiota by Trichuris suis infection. PLoS One. 2012; 7:e35470.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download