Oseltamivir is a neuraminidase inhibitor that is widely used in prophylaxis and in the treatment of influenza A and B viral infections (
123). From a pharmacokinetic perspective, oseltamivir is readily absorbed with an oral bioavailability of more than 80%. It is extensively hydrolyzed in the liver to its active metabolite, oseltamivir carboxylate. Oseltamivir carboxylate reaches peak concentrations after 3–4 hous, exceeding the systemic exposure of oseltamivir by > 20-fold. It is eliminated mainly by renal route with an elimination half-life of 6–10 hours (
34). From a safety perspective, the causal relationships between oseltamivir administration and neuropsychiatric adverse events have been continuously issued up to date, although several studies of neuropsychiatric adverse events with oseltamivir has not established a direct causal relationship (
12567). The US Food and Drug Administration's adverse events reporting system has warned of high odds ratio scores in oseltamivir-associated neuropsychiatric adverse events such as abnormal behavior (29.35), psychiatric and behavioral symptoms (15.36), delirium (13.50), and hallucinations (12.00) (
8).
P-glycoprotein (P-gp) is expressed in the intestinal epithelium, liver, kidney, and blood-brain barrier (BBB) as an efflux transporter by pumping out substrates and resulting in altered substrates' pharmacokinetic characteristics (
910). A series of preclinical studies were conducted to explore the role of P-gp, which is expressed in the BBB, on the distribution of oseltamivir in the brain. In vitro and in vivo studies suggest that oseltamivir might be a substrate of P-gp, encoded by adenosine triphosphate (ATP)-binding cassette subfamily B member 1 (
ABCB1) gene. By contrast, oseltamivir carboxylate transport by P-gp was negligible in preclinical studies (
12).
The functional activity of
ABCB1 polymorphisms c.1236C>T, c.2677G>T/A, c.3435C>T, and their substrates' pharmacokinetics have been studied widely, since
ABCB1 polymorphisms are related to dysfunction of P-gp (
91011121314). In this context, we hypothesized that
ABCB1 polymorphisms might alter systemic exposure to oseltamivir. However, the effect of
ABCB1 polymorphisms on the pharmacokinetics of oseltamivir in humans has not been studied. Therefore, we assessed the potential role of
ABCB1 polymorphisms in altering the pharmacokinetics of oseltamivir.
In total, 19 healthy individuals were enrolled. The study was conducted in accordance with the principles enshrined in the Declaration of Helsinki as well as Good Clinical Practice. All participants were given a 75 mg single oral dose of oseltamivir (Tamiflu®; Roche Korea, Seoul, Korea). Blood samples were collected at scheduled times during the study period: prior to dosing (0 hours), 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, and 48 hours after drug administration. Blood samples were collected in fluoride-oxalate tubes. Plasma was separated by centrifugation at 1,700 g and 4°C for 15 minutes and stored at −70°C until drug concentration analysis was performed. A blood sample was drawn from each individual for genetic analysis and stored in ethylenediaminetetraacetic acid (EDTA) at −20°C until DNA extraction was performed.
The plasma concentrations of oseltamivir and oseltamivir carboxylate were analyzed by validated liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) method after protein precipitation with acetonitrile. In brief, plasma samples (0.1 mL) were spiked with an internal standard (10 μL of oseltamivir-d3 300 ng/mL, and 10 μL of oseltamivir-d3 carboxylate 4 μg/mL), and 500 μL of methanol. After vigorous vortex mixing, 100 μL of deionized water was added to the 100 μL of supernatant. A 3 μL aliquot of this solution was injected into the LC (Shimadzu Prominence LC; Shimadzu Co., Kyoto, Japan) and detected by an MS/MS (API5000; AB SCIEX, Foster city, CA, USA) system. Chromatographic separation of the compound was conducted using a T3 column (1.8 μm, 2.1 × 50 mm) with a mobile phase consisting of acetonitrile including 0.1% formic acid. The mass spectrometer with an electrospray source was run in the positive mode and m/z was 313.25 → 225.1 for oseltamivir, 316.25 → 228.1 for oseltamivir-d3, 285.25 → 197.1 for oseltamivir carboxylate, and 288.25 → 200 for oseltamivir-d3 carboxylate. The lower limits of quantification for oseltamivir and oseltamivir carboxylate were 1 ng/mL and 10 ng/mL. Linearity calibration curves were established within the ranges of 1−200 ng/mL for oseltamivir and 10−2,000 ng/mL for oseltamivir carboxylate (coefficients of determination: r2
≥ 0.9950). The respective accuracy levels of oseltamivir and oseltamivir carboxylate ranged from 84.1%−121.4% and 91.4%−114.4%. The respective precision levels of oseltamivir and oseltamivir carboxylate, expressed as coefficients of variation, were 4.4% and 2.6%.
DNA was extracted using standard methods (QIAamp DNA Blood Mini Kit; Qiagen, Hilden, Germany).
ABCB1 polymorphisms c.1236C>T, c.2677G>T/A, and c.3435C>T were genotyped in all subjects by pyrosequencing methods using a Pyrosequencer (Biotage, Uppsala, Sweden), as referenced from a previous study (
13). The validity of method was confirmed by direct sequencing.
The pharmacokinetic variables of oseltamivir were estimated by non-compartmental methods using Phoenix
® version 6.4 (Certara USA Inc., St. Louis, MO, USA). The peak drug concentrations (C
max) and the time to reach C
max (T
max) were obtained. The area under the plasma concentration-time curve (AUC) from time zero to infinity (AUC
inf) was calculated using the formula:
Ct is the final recorded plasma concentration, ke is the elimination rate constant as determined by the linear regression of the log-linear part of the plasma concentration-time curve, and AUClast is the AUC from time zero to the final measurable time, derived using the linear trapezoidal rule from 0 to 24 hours for oseltamivir and 0 to 48 hours for oseltamivir carboxylate. The elimination half-life (t1/2) was calculated as 0.693/ke.
Statistical comparisons of pharmacokinetic variables were performed using the Kruskal-Wallis tests, followed by the Mann-Whitney U test involving post hoc comparisons with a Bonferroni correction. Differences were considered statistically significant when the P value was below 0.05 for the Kruskal-Wallis test and the P value was below 0.017 for the Mann-Whitney U test involving post hoc comparisons with a Bonferroni correction. The data were analyzed using IBM SPSS 19.0 for Windows (IBM Corp., Chicago, IL, USA).
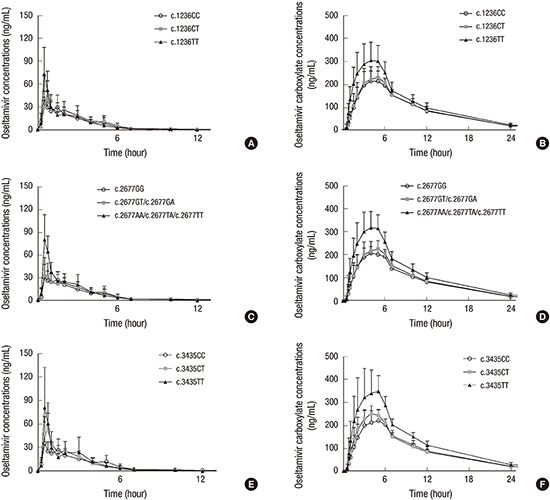
The mean oseltamivir and oseltamivir carboxylate plasma concentration profiles for each group are illustrated in
Fig. 1. Systemic exposure to oseltamivir and oseltamivir carboxylate was higher in the mutant group than in the wild-type and heterozygous variant groups. For oseltamivir, C
max and AUC
inf measured in
ABCB1 c.2677G>T/A mutant group were 88%–92% and 26%–36% higher than that measured in the wild-type and heterozygous groups (
P = 0.030 and
P = 0.034, respectively). For oseltamivir carboxylate, C
max and AUC
inf measured in
ABCB1 c.1236C>T mutant group were 31%–41% and 27%–30% higher than that measured in the wild-type and heterozygous groups (
P = 0.040 and
P = 0.052, respectively). With regard to
ABCB1 c.2677G>T/A polymorphism, C
max and AUC
inf measured in mutant group were 37%–58% and 34%–41% higher than that measured in the wild-type and heterozygous groups (
P = 0.002 and
P = 0.009, respectively). In terms of
ABCB1 c.3435C>T polymorphism, C
max and AUC
inf measured in mutant group were 40%−59% and 40%−46% higher than that measured in the wild-type and heterozygous groups (
P = 0.021 and
P = 0.034, respectively) (
Tables 1,
2,
3). No serious adverse events were observed in the current study.
In this study, we provide evidence of a partial contribution of ABCB1 polymorphisms to the pharmacokinetics of oseltamivir in humans. We found that the systemic exposure to oseltamivir and oseltamivir carboxylate was increased in the mutant group compared to other groups.
The increased systemic exposure to oseltamivir in the mutant group may relate to the findings that oseltamivir is a substrate of P-gp (
12) and that
ABCB1 polymorphisms are associated with lower intestinal P-gp expression and function (
1213), which results in elevated blood concentration of its substrates. Namely, we suggest that dysfunction of intestinal P-gp in the mutant group play a role in the increased absorption of oseltamivir since oseltamivir extensively metabolized to oseltamivir carboxylate, and there is minimal renal excretion of unchanged oseltamivir (< 5%) (
4).
Notably, increased systemic exposure to oseltamivir carboxylate in the mutant group was also observed despite evidence from preclinical studies, which showed that BBB transfer of oseltamivir carboxylate was not influenced by P-gp, indicating that oseltamivir carboxylate is not a substrate of P-gp (
12). A plausible explanation for this contradictory result is that the differences in oseltamivir exposure between the mutant group and the other two groups was expected to be manifested in oseltamivir carboxylate exposure differences. This assumption is based on the facts that absorbed oseltamivir is extensively bio-transformed to oseltamivir carboxylate and that the AUC
inf of oseltamivir carboxylate is much greater (> 20-fold) than that of oseltamivir (
3).
The increased systemic exposure to oseltamivir and oseltamivir carboxylate in the mutant group compared to other groups could be important to the safety profiles of oseltamivir. Recently, Morimoto et al. (
15) and Nakamura et al. (
16) reported that the partial contributions of enhanced serum or plasma concentrations of oseltamivir carboxylate on the neuropsychiatric adverse events that occurred after oseltamivir administration in influenza patients. In addition, a clinical study was conducted to explore the role of
ABCB1 polymorphisms on the incidence of neuropsychiatric adverse events in influenza-infected children treated with oseltamivir. An increased incidence of neuropsychiatric adverse events was reported in
ABCB1 c.2677G>T/A-c.3435C>T diplotype mutant group compared to wild-type and heterozygous variant groups (
P = 0.149) (
17). Oseltamivir dosages of up to 675 mg were well-tolerated in healthy subjects without drug-related neuropsychiatric adverse events (
18). Considering the high safety margin of oseltamivir in clinical studies, there is no need for dose adjustment to avoid unexpected adverse events related to increased oseltamivir or oseltamivir carboxylate exposure according to the
ABCB1 polymorphisms. However, we cannot exclude the possibility that increased systemic exposure to oseltamivir in the
ABCB1 mutant group is one of the factors causing the modified distribution of oseltamivir in the brain that leads to neuropsychiatric adverse events occurring in some people following oseltamivir intake. Particularly in the light of the findings from a clinical study that indicate C
max cerebrospinal fluid (CSF)/plasma ratios of 2.1% for oseltamivir and 3.5% for oseltamivir carboxylate, AUC CSF/plasma ratios of 2.4% for oseltamivir and 2.9% for oseltamivir carboxylate (
19).
This study has some limitations. Firstly, this study was conducted in a small population. Although differences in the systemic exposure to oseltamivir and oseltamivir carboxylate between groups were statistically significant, each group consisted of less than 10 individuals. Secondly, this study was conducted in healthy subjects. However, pharmacokinetic values have been shown to be similar in healthy subjects and influenza patients, encouraging speculation that similar results can be expected in influenza patients (
3). Thirdly, this was a single-dose study. However, the pharmacokinetic values were comparable after single-dose and multiple-dose (twice-daily for 7 days) administration of oseltamivir (50−100 mg), except for the accumulation index of oseltamivir carboxylate, which ranged from 1.57 to 1.74, indicating that oseltamivir carboxylate was accumulated after multiple-dose administration of oseltamivir (
20). Therefore, the pharmacokinetic variations of oseltamivir carboxylate between the
ABCB1 mutant group and the other groups observed in this study would be much greater in clinical settings, in which multiple-dose (twice-daily dose of 75 mg for 5 days) administration of oseltamivir is prescribed.
Our findings suggest that ABCB1 polymorphisms are factors influencing the pharmacokinetics of oseltamivir in the body. Further studies in a large population are necessary to confirm the results of this preliminary study.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download