INTRODUCTION
CASE DESCRIPTION
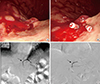
Case 1
Fig. 2
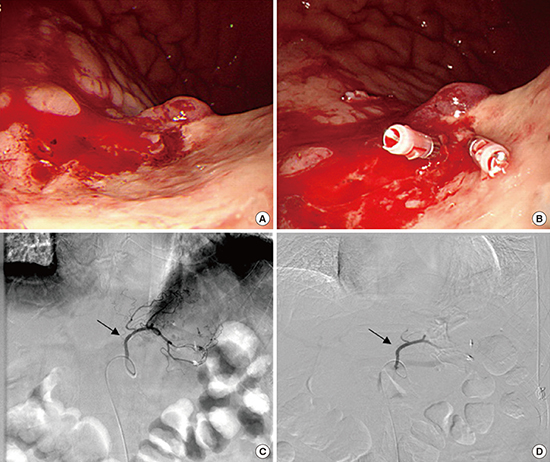
Case 2
Fig. 3
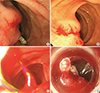
Fig. 4
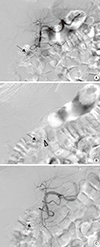
DISCUSSION
Table 1
Baseline and clinical characteristics for each case undergoing TAE

Journal List > J Korean Med Sci > v.32(9) > 1108440




Ko Eun Lee 
https://orcid.org/0000-0002-1261-5702
Ki-Nam Shim 
https://orcid.org/0000-0003-4004-6292
Chung Hyun Tae 
https://orcid.org/0000-0002-0764-7793
Min Sun Ryu 
https://orcid.org/0000-0003-2613-4501
Sun Young Choi 
https://orcid.org/0000-0003-2488-1183
Chang Mo Moon 
https://orcid.org/0000-0003-2550-913X
Seong-Eun Kim 
https://orcid.org/0000-0002-6310-5366
Hey-Kyung Jung 
https://orcid.org/0000-0002-6653-5214
Sung-Ae Jung 
https://orcid.org/0000-0001-7224-2867