1. Laohaburanakit P, Hardin KA. NK/T cell lymphoma of the lung: a case report and review of literature. Thorax. 2006; 61:267–270.
2. Morovic A, Aurer I, Dotlic S, Weisenburger DD, Nola M. NK cell lymphoma, nasal type, with massive lung involvement: a case report. J Hematop. 2010; 3:19–22.
3. Al-Hakeem DA, Fedele S, Carlos R, Porter S. Extranodal NK/T-cell lymphoma, nasal type. Oral Oncol. 2007; 43:4–14.
4. Tse E, Liang RH. Natural killer cell neoplasms. Clin Lymphoma. 2004; 5:197–201.
5. Kanavaros P, Lescs MC, Brière J, Divine M, Galateau F, Joab I, Bosq J, Farcet JP, Reyes F, Gaulard P. Nasal T-cell lymphoma: a clinicopathologic entity associated with peculiar phenotype and with Epstein-Barr virus. Blood. 1993; 81:2688–2695.
6. Chim CS, Ma SY, Au WY, Choy C, Lie AK, Liang R, Yau CC, Kwong YL. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood. 2004; 103:216–221.
7. Suzuki R, Takeuchi K, Ohshima K, Nakamura S. Extranodal NK/T-cell lymphoma: diagnosis and treatment cues. Hematol Oncol. 2008; 26:66–72.
8. Aozasa K, Takakuwa T, Hongyo T, Yang WI. Nasal NK/T-cell lymphoma: epidemiology and pathogenesis. Int J Hematol. 2008; 87:110–117.
9. Cheung MM, Chan JK, Wong KF. Natural killer cell neoplasms: a distinctive group of highly aggressive lymphomas/leukemias. Semin Hematol. 2003; 40:221–232.
10. Lee J, Kim WS, Park YH, Park SH, Park KW, Kang JH, Lee SS, Lee SI, Lee SH, Kim K, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005; 92:1226–1230.
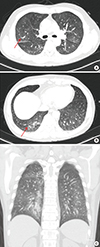
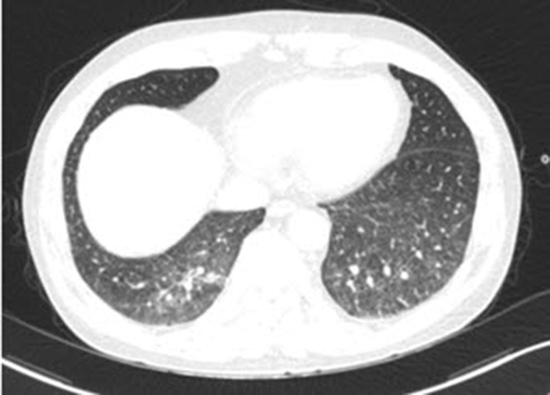
11. Lee BH, Kim SY, Kim MY, Hwang YJ, Han YH, Seo JW, Kim YH, Cha SJ, Hur G. CT of nasal-type T/NK cell lymphoma in the lung. J Thorac Imaging. 2006; 21:37–39.
12. Oshima K, Tanino Y, Sato S, Inokoshi Y, Saito J, Ishida T, Fukuda T, Watanabe K, Munakata M. Primary pulmonary extranodal natural killer/T-cell lymphoma: nasal type with multiple nodules. Eur Respir J. 2012; 40:795–798.
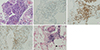
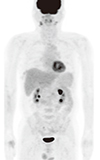
13. Fei W, Xiaohong W, Hong Z, Bei H. Pulmonary extranodal natural killer/T-cell lymphoma (nasal type): a case report and radiological image review. Medicine (Baltimore). 2015; 94:e1527.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download