INTRODUCTION
Implantable cardioverter defibrillators are indicated in patients at high risk of sudden cardiac death and in those who have survived sudden cardiac death due to fatal ventricular arrhythmia. In prior studies, these devices have been shown to be superior to antiarrhythmic agents in primary or secondary prevention of sudden cardiac death (
123). Different companies have developed various lead designs for their devices, some of which have been reported to have higher lead failure rates than others (
45). The Riata defibrillator leads, manufactured by St. Jude Medical, Inc. (St. Paul, MN, USA), were designated to class I recall by the Food and Drug Administration in December 2011 due to substantial rates of lead failure and insulation defect. These leads had a silicone-only insulation design, which contributed to conductor externalization. The association between externalized conductor (EC) and electrical dysfunction (ED) has not been previously established (
67), and prior studies have suggested that the recalled Riata defibrillator leads might be more difficult to extract than others (
89). Therefore, the routine removal of recalled Riata leads has not been recommended. Approximately 500 Riata leads were implanted between 2003 and 2011 in Korea. However, there is a paucity of data regarding Riata lead failure. Therefore, we investigated the incidence of Riata defibrillator lead failure at Samsung Medical Center.
Go to :

MATERIALS AND METHODS
We enrolled consecutive patients who received implantable cardioverter defibrillator implantation or underwent cardiac resynchronization therapy with Riata® or Riata® ST silicone defibrillation leads at our center between January 2003 and December 2010. A trained study coordinator used a standardized case report form and protocol to collect the clinical and laboratory outcome data.
Routine chest X-rays were performed in the posterior-anterior and lateral views on the first visit day after lead implantation. Follow-up chest X-rays were performed at the physicians' discretion. We defined EC when a conductor was visibly displaced from the lead body. EC localization was categorized into the following four zones: A, distal to the superior vena cava (SVC) coil (dual coil lead) or distal to the SVC (single coil leads); B, distal to the clavicle, including the SVC coil (dual coil lead) or SVC area (single coil); C, clavicular area; and D, distal clavicle to the generator can. Location A was subcategorized into three zones: A1, proximal right ventricular coil to the tricuspid annulus; A2, tricuspid annulus; and A3, tricuspid annulus to the SVC coil for dual coils or right atrium for single coil leads (
Fig. 1A and 1B) (
6).
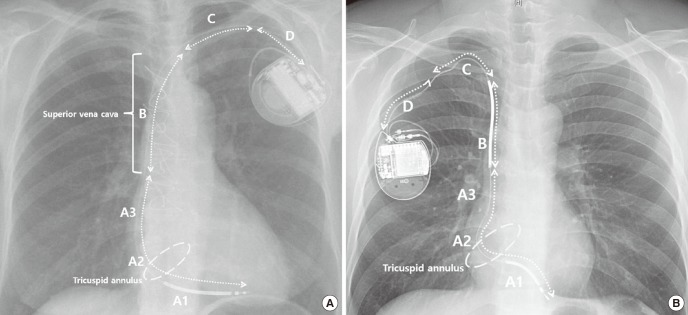 | Fig. 1
Location of conductor externalization on chest X-ray (posterior-anterior view). (A) Single coil lead. (B) Dual coil lead.
A = distal to the SVC coil (dual coil lead) or distal to the SVC (single coil lead) (A1, proximal right ventricular coil to the tricuspid annulus; A2, tricuspid annulus; A3, tricuspid annulus to the SVC coil [dual coil lead] or right atrium [single coil lead]), B = distal to the clavicle including the SVC coil (dual coil lead) or SVC area (single coil lead), C = clavicular area, D = distal clavicle to the generator can, SVC = superior vena cava.

|
Device interrogation was performed by measuring the R wave amplitude, pacing and shock impedances, and sensing and pacing threshold of lead. ED was defined when any of the following criteria was met: 1) non-physiologic signals on intracardiac electrogram (noise sensing); 2) R wave < 3.0 mV or 50% reduction; 3) pacing impedance < 200 or >2,000 Ω or high-voltage impedance > 200 or < 25 Ω; or 4) pacing threshold > 5 V or > 100% increase (
6). Concurrent episodes associated with lead failure were also investigated, including inappropriate or no shock therapy. Reason for lead replacement or extraction, procedural success, and procedure complications were investigated.
Categorical variables are represented by frequencies and percentages and were compared using the χ2 test. Continuous variables are shown as medians with interquartile ranges and were compared using the Mann-Whitney U test or the Kruskal-Wallis test. The incident rate of EC was evaluated using the time at risk at < 3 years, 3–5 years, and > 5 years after lead implantation. The incidence of ED was evaluated every year after lead implantation. The survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. All tests were two sided. P values less than 0.05 were considered statistically significant. Cox regression model was used to determine the independent predictors of ED. Variables with P value < 0.2 in univariate analysis were included in the multivariate analysis. All statistical analyses were performed using IBM SPSS Statistics Version 23 (IBM Corporation, Armonk, NY, USA).
Ethics statement
The Institutional Review Board at Samsung Medical Center approved the study protocol (IRB No. 2016-11-028). Informed consent was waived by the board.
Go to :

RESULTS
Five of the 49 consecutive patients were excluded due to loss to follow-up after device implantation (n = 2) or the absence of a follow-up chest X-ray (n = 3) (
Fig. 2). The baseline patient characteristics are shown in
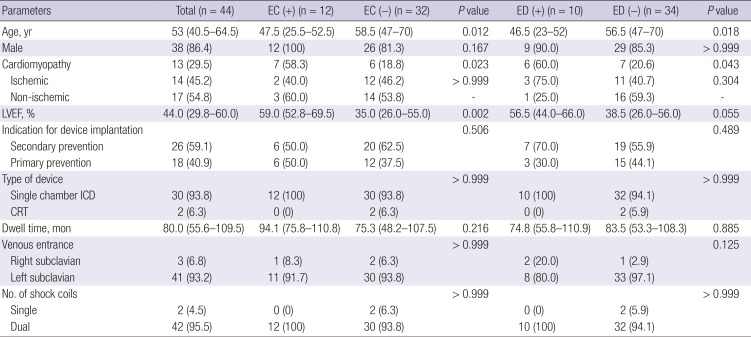
Table 1. All device generators were implanted in the subcutaneous pocket. Majority of patients received a single chamber device (93.8%) or dual shock coil leads (95.5%). Most of the leads were inserted via the left subclavian vein (93.2%). The median lead dwell time was 80.0 (55.6–109.5) months. Patients with EC were significantly younger than those without EC (47.5 vs. 58.5 years,
P = 0.012). Those with EC also had a significantly higher prevalence of cardiomyopathy (58.3% vs. 18.8%,
P = 0.023) and higher left ventricular ejection fraction (LVEF) than did those without EC (59.0% vs. 35.0%,
P = 0.002). Similarly, patients with ED were significantly younger (46.5 vs. 56.5 years,
P = 0.018) and had a higher prevalence of cardiomyopathy than did those without ED (60.0% vs. 20.6%,
P = 0.043).
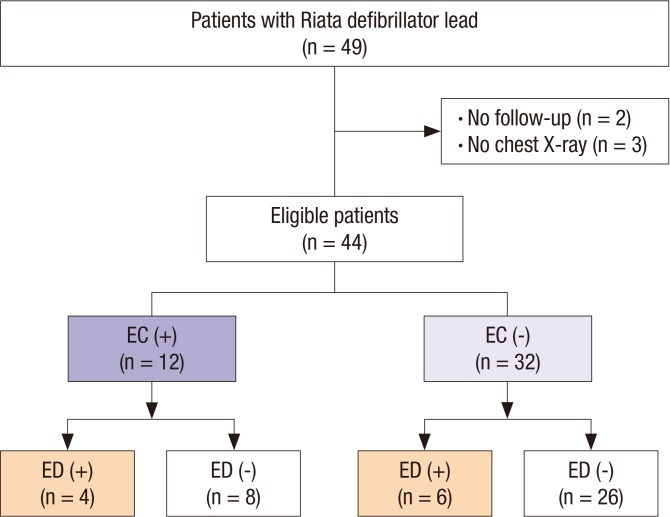 | Fig. 2
Flow diagram of study patients.
EC = externalized conductor, ED = electrical dysfunction.

|
Table 1
Comparisons of clinical characteristics according to the presence of EC or ED
|
Parameters |
Total (n = 44) |
EC (+) (n = 12) |
EC (−) (n = 32) |
P value |
ED (+) (n = 10) |
ED (−) (n = 34) |
P value |
|
Age, yr |
53 (40.5–64.5) |
47.5 (25.5–52.5) |
58.5 (47–70) |
0.012 |
46.5 (23–52) |
56.5 (47–70) |
0.018 |
|
Male |
38 (86.4) |
12 (100) |
26 (81.3) |
0.167 |
9 (90.0) |
29 (85.3) |
> 0.999 |
|
Cardiomyopathy |
13 (29.5) |
7 (58.3) |
6 (18.8) |
0.023 |
6 (60.0) |
7 (20.6) |
0.043 |
|
Ischemic |
14 (45.2) |
2 (40.0) |
12 (46.2) |
> 0.999 |
3 (75.0) |
11 (40.7) |
0.304 |
|
Non-ischemic |
17 (54.8) |
3 (60.0) |
14 (53.8) |
- |
1 (25.0) |
16 (59.3) |
- |
|
LVEF, % |
44.0 (29.8–60.0) |
59.0 (52.8–69.5) |
35.0 (26.0–55.0) |
0.002 |
56.5 (44.0–66.0) |
38.5 (26.0–56.0) |
0.055 |
|
Indication for device implantation |
|
|
|
0.506 |
|
|
0.489 |
|
Secondary prevention |
26 (59.1) |
6 (50.0) |
20 (62.5) |
7 (70.0) |
19 (55.9) |
|
Primary prevention |
18 (40.9) |
6 (50.0) |
12 (37.5) |
3 (30.0) |
15 (44.1) |
|
Type of device |
|
|
|
> 0.999 |
|
|
> 0.999 |
|
Single chamber ICD |
30 (93.8) |
12 (100) |
30 (93.8) |
10 (100) |
32 (94.1) |
|
CRT |
2 (6.3) |
0 (0) |
2 (6.3) |
0 (0) |
2 (5.9) |
|
Dwell time, mon |
80.0 (55.6–109.5) |
94.1 (75.8–110.8) |
75.3 (48.2–107.5) |
0.216 |
74.8 (55.8–110.9) |
83.5 (53.3–108.3) |
0.885 |
|
Venous entrance |
|
|
|
> 0.999 |
|
|
0.125 |
|
Right subclavian |
3 (6.8) |
1 (8.3) |
2 (6.3) |
2 (20.0) |
1 (2.9) |
|
Left subclavian |
41 (93.2) |
11 (91.7) |
30 (93.8) |
8 (80.0) |
33 (97.1) |
|
No. of shock coils |
|
|
|
> 0.999 |
|
|
> 0.999 |
|
Single |
2 (4.5) |
0 (0) |
2 (6.3) |
0 (0) |
2 (5.9) |
|
Dual |
42 (95.5) |
12 (100) |
30 (93.8) |
10 (100) |
32 (94.1) |

Fig. 3 demonstrates the incidence of EC based on follow-up chest X-ray. The highest incidence of EC was detected 3–5 years after lead implantation (44.4% vs. 18.2% at < 3 years vs. 25.0% at > 5 years) (
Fig. 3). EC only occurred in patents with a dual coil lead. The most common site of EC was the A2 area (n = 9, 75%).
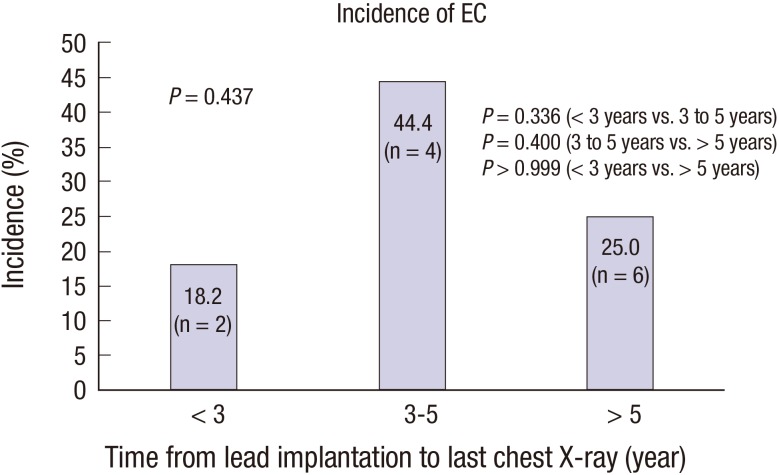 | Fig. 3
Incidence of EC of Riata leads on follow-up chest X-ray.
EC = externalized conductor.

|
Device interrogation was performed at least annually in all patients. The total cumulative incidence of ED was 22.7% (n = 10). There was a bimodal incidence of ED according to dwell time, with peaks at 5 years and 9 years (
Fig. 4). There was no difference in survival rate free from ED according to the presence of EC (
Fig. 5). Three patients with noise sensing experienced inappropriate shocks. Different types of electrical lead dysfunction are shown in
Fig. 6.
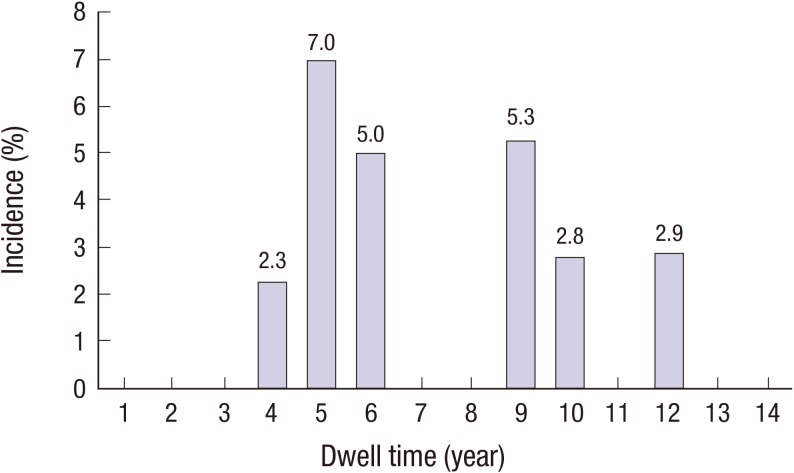 | Fig. 4
The incidence of ED according to dwell time.
ED = electrical dysfunction.

|
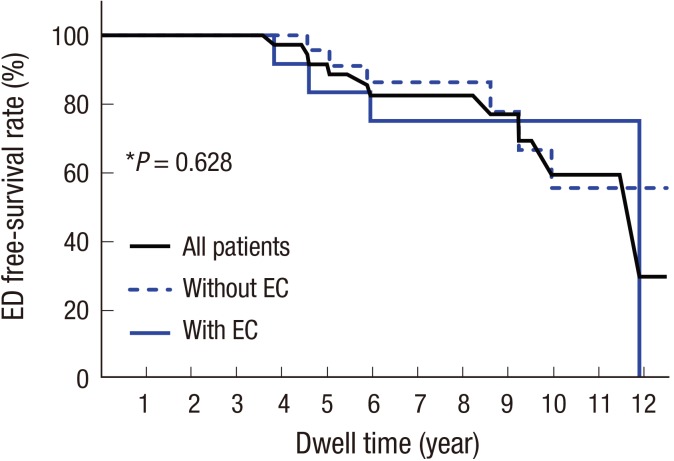 | Fig. 5
Kaplan-Meier curve of cumulative probabilities of ED-free survival according to the EC of Riata leads.
*P value refers to the result of the log-rank test between patients with and without ECs.
EC = externalized conductor, ED = electrical dysfunction.

|
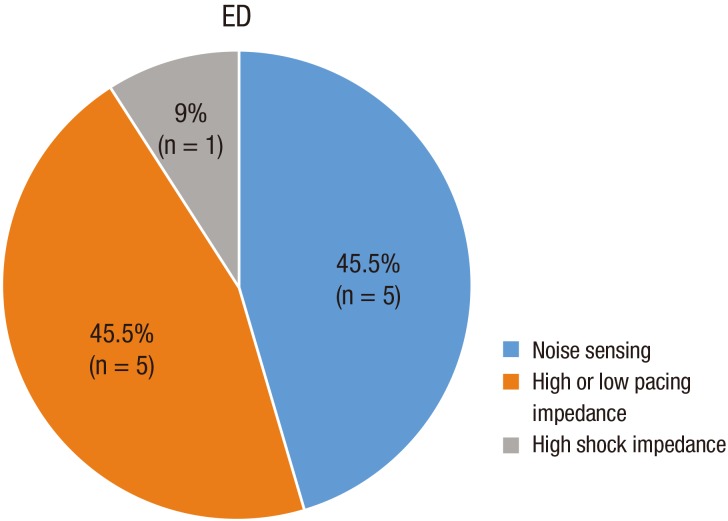 | Fig. 6
Different types of electrical lead dysfunction.
ED = electrical dysfunction.

|
Cox regression analysis was performed to determine the prognostic factors for ED. In univariate analysis, higher LVEF, absence of underlying cardiomyopathy, and right subclavian access were all significantly associated with ED. However, there were no significant predictors of ED in multivariate analysis (
Table 2).
Table 2
Predictors of ED
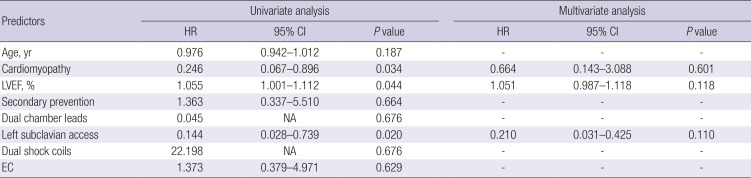
|
Predictors |
Univariate analysis |
Multivariate analysis |
|
HR |
95% CI |
P value |
HR |
95% CI |
P value |
|
Age, yr |
0.976 |
0.942–1.012 |
0.187 |
- |
- |
- |
|
Cardiomyopathy |
0.246 |
0.067–0.896 |
0.034 |
0.664 |
0.143–3.088 |
0.601 |
|
LVEF, % |
1.055 |
1.001–1.112 |
0.044 |
1.051 |
0.987–1.118 |
0.118 |
|
Secondary prevention |
1.363 |
0.337–5.510 |
0.664 |
- |
- |
- |
|
Dual chamber leads |
0.045 |
NA |
0.676 |
- |
- |
- |
|
Left subclavian access |
0.144 |
0.028–0.739 |
0.020 |
0.21 |
0.031–0.425 |
0.11 |
|
Dual shock coils |
22.198 |
NA |
0.676 |
- |
- |
- |
|
EC |
1.373 |
0.379–4.971 |
0.629 |
- |
- |
- |

The relevant data are summarized in
Table 3. A total of 13 (29.5%) patients had their leads replaced or extracted, with 6 patients in the EC (+) group and 7 in the EC (−) group. Among the six patients with EC, two underwent elective lead replacement while the generator was being exchanged for battery depletion. The rates of lead replacement due to ED were comparable between the two patient groups with or without EC (
P = 0.422). Lead extraction was attempted in 9 patients (n = 3 in the EC [+] group, and n = 6 in the EC [−] group). ED was the indication for replacement in every case, with the exception of one patient who developed a device-related infection. The extraction failed in two patients with ED, but in none with EC; therefore, the extraction success rate was 77.8%. There were no significant complications related to lead replacement or extraction.
Table 3
Results of lead extraction
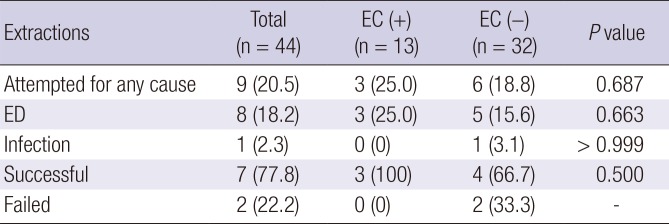
|
Extractions |
Total (n = 44) |
EC (+) (n = 13) |
EC (−) (n = 32) |
P value |
|
Attempted for any cause |
9 (20.5) |
3 (25.0) |
6 (18.8) |
0.687 |
|
ED |
8 (18.2) |
3 (25.0) |
5 (15.6) |
0.663 |
|
Infection |
1 (2.3) |
0 (0) |
1 (3.1) |
> 0.999 |
|
Successful |
7 (77.8) |
3 (100) |
4 (66.7) |
0.500 |
|
Failed |
2 (22.2) |
0 (0) |
2 (33.3) |
- |

Go to :

DISCUSSION
In the present study, we investigated the incidence of ED, EC, and replacement of Riata leads. The major findings of our study were as follows: 1) The total cumulative incidences of EC and ED were 27.3% and 22.7%, respectively, during a median dwell time of 80 months; 2) EC were most frequently detected in patients who underwent X-ray analysis 3–5 years after lead implantation, while the incidence of ED demonstrated a bimodal pattern, with peaks at 5 years and 9 years. Interestingly, there was no association between EC and ED; and 3) finally, the success rate of lead extraction was 77.8% without any significant complications.
The US Food and Drug Administration issued a class I recall for Riata leads due to premature insulation abrasion in December 2011. Approximately 500 Riata leads were implanted in Korea between 2003 and 2011, prior to its recall. We previously reported the first case of lead failure, which was characterized by an externalized Riata lead that generated an inappropriate shock due to noise sensing (
10). To date, there has not been a large study addressing the incidence of Riata lead failure in Korea. Therefore, this is the first study that investigates the fate of Riata leads in Korea. In this study, the EC detection rate was highest 3–5 years after lead implantation. This result was discordant with time-dependent EC, which has been described in previous studies (
45). In one study, patients underwent fluoroscopic screening, and the externalization rate reached 26% at ≥ 5 years after lead implantation (
4). In contrast, the EC rate in our study was 44.4% at 3–5 years. Our finding was substantially higher than the prior results not only with regard to its numerical value, but also to the fact that EC was detected by simple radiography, which is less sensitive than fluoroscopy. This discrepancy might be partially explained by inclusion of patients with 8 Fr leads, which are known to have higher EC rates than 7 Fr leads (
1112). The A2 area was the most common site of EC in this study, which is consistent with previous findings (
6). The mechanism of mechanical failure of Riata leads involves their unique design, which is silicon-only insulation and hollow multilumen. This would facilitate movement of internal conductors exerting pressure on the inner luminal surface of the lead, which resulted in inside-out abrasion. There are known mechanisms for lead failure: subclavian crush syndrome, abrasion of the lead by the implantable cardioverter-defibrillator (ICD) generator, or abrasion of the lead at the level of lead fixation. Additionally, mechanical stress of tricuspid leaflet was reported as a potential cause of lead disintegration (
13). Considering the mechanism of EC in Riata leads, it is likely that tricuspid leaflet attributed further movement of conductors located in A zone.[REMOVED HYPERLINK FIELD] Even though direct comparison between the studies is limited due to different study design and follow-up duration, the incidence of ED was comparable with previous studies (
45).
We did not find any correlation between EC and ED, which is also consistent with prior study results (
67). Given the lack of association between structural and functional failure of Riata leads, their routine replacement is not recommended regardless of the presence of EC, unless there is evidence of ED. However, one prior study proposed a possible relationship between EC and ED (
5). The authors suggest that previous studies were prone to survival bias resulting from their retrospective and cross-sectional study designs. They found a correlation between EC and ED with an adjusted incidence rate ratio of 4.4 and cited additional supporting studies (
1214). Regardless of the residual controversy, we postulate that the mechanism of ED in leads without EC might be that mechanical disintegration of leads which could not be detected by X-ray including conductor fracture or very subtle EC.
Prior studies have suggested that removing Riata leads would be more challenging than removing other leads because of EC and the absence of coil backfill (
9). Therefore, prophylactic extraction has been discouraged due to the risk of complications. However, several large-volume centers have reported a high success rate and low complication rate of Riata lead extraction (
1516). In addition to the potential correlation between ED and EC and the excellent clinical success of Riata lead extraction at experienced centers, the optimal management for Riata leads needs more consideration. Lead failure is a potentially life-threatening issue and can be more problematic for young patients, because EC is known to be time-dependent, and ED can occur even a long time after lead implantation. Therefore, there is a need for individualized approaches for Riata lead management according to age, morbidity, potential risk after lead failure, and experience of lead extraction at each center.
This study has several limitations. This was a retrospective, single-center study with a small number of patients. Therefore, it is underpowered to perform multivariate analysis for the predictors of ED. Second, there is a chance that the incidence of EC was underestimated because it was detected by simple radiography rather than fluoroscopy. Furthermore, EC was defined by only a clear separation of conductor cable from the lead body. In addition, follow-up of chest X-ray was left to the physicians' discretion without standardized protocol. Lastly, we only included patients with 8 Fr Riata lead, however, it was because 7 Fr leads were not imported in Korea.
In conclusion, long-term close monitoring is mandatory for patients with Riata defibrillator leads considering the delayed occurrence of ED and residual controversy over the association between EC and ED. An individualized approach is required for optimal management of Riata leads according to patient characteristics, potential risk after lead failure, and the experience of lead extraction at each respective center.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download