Abstract
A group of benign Theileria species, which are often referred to as T. orientalis/T. buffeli/T. sergenti group, has low pathogenicity in cattle. Herein, we report on Theileria spp. in cattle on a farm from China. Based on phylogenetic analysis of the major piroplasm surface protein gene sequences, we detected 6 genotypes that were categorized as Types 1, 2, 3, 4, and 5 as well as an additional Type 9 genotype. The new epidemiological features of the T. orientalis/T. buffeli/T. sergenti parasites in China indicate a greater diversity in the genetics of these species than had been previously thought.
A group of benign Theileria species transmitted by Haemaphysalis ticks is often referred to as T. orientalis/T. buffeli/T. sergenti group. The parasite's presence is characterized by anemia, jaundice, depression, abortion, mortality, and the presence of Theileria in blood films [4]. Benign Theileria group parasites are widespread among cattle in subtropical and temperate zones [6], and the parasite can eventually lead to severe economic losses in endemic areas. For simplicity, T. orientalis is used throughout this paper to denote this benign Theileria group. Herein, we report on Theileria spp. in cattle on a farm in the Shaanxi Province, a region in which disease outbreaks have occurred.
The exact taxonomic status of T. orientalis has been debated for many years. The 18S rRNA data have shown that parasites in this group can be divided into at least 8 types, A, B, B1, C, D, E, H, and T. buffeli (Warwick). However, the number of identified types is increasing and there is a lack of consensus on their nomenclature [1213]. Recently, the gene encoding the major piroplasm surface protein (MPSP) was considered a highly useful marker in revealing the phylogeny of Theileria parasites [3].
The nomenclature for the benign Theileria group has not reached consensus to date. In 1995, Kubota et al. [7] divided the group into at least four types, Ikeda (I), Chitose (C), Thai, and Buffeli (B), based on the allelic forms of the MPSP gene. However, Kim et al. [6] proposed a different classification approach based on MPSP, in which there were 6 genotypes, designated Types 1 to 6. Subsequently, 5 additional genotypes, including Types 7, 8, and N1 to N3, were added to the list of previously described genotypes [35].
Theileriosis caused by the benign Theileria group is widely reported from countries neighboring China. However, there are few reports on the occurrence of benign Theileria in China. Liu et al. [8] and He et al. [2] have reported that Theileria spp. are present in Hubei Province of China. In addition, surveys have revealed that at least five genotypes of T. orientalis (Types 2, 3, 6, 7, and 8) exist in China [3].
This study investigated the genetic diversity of the benign Theileria parasites in Huanglong county, which is located in the northern part of Shaanxi Province, China, by analyzing blood samples gathered from cattle at a farm where there was an outbreak of clinical piroplasmosis following a severe tick infestation (species unknown) in August 2013. Blood samples were collected from 10 cattle showing appropriate clinical signs: marked pallor in the oral and genital mucosa, anorexia, and fever (rectal temperature with high readings ≤ 42℃). The diagnoses were made based on clinical signs and a light microscopic examination of Giemsa-stained blood smears. To confirm the species of the piroplasm, we conducted polymerase chain reaction (PCR) with primer set 989/990 [11] from the 18S rRNA gene sequences of Theileria species. Subsequently, 5 randomly selected PCR-positive samples were cloned and sequenced. In order to further identify the types of Theileria present, the MPSP genes were examined according to a previously described method [10] using MPSP-specific primers from the T. orientalis group. All PCR products were cloned and sequenced for confirmation by performing sequencing analysis.
One type of polymorphic Theileria-like parasite was identified in the erythrocytes in the anticoagulant-treated blood samples by performing light microscopy-based examinations. Dot-shaped, pyriform or irregular-shaped parasites were seen, and there were generally 2 to 4 parasites per erythrocyte (Fig. 1). The rate of parasitemia reached 13.7%.
The obtained 18S rRNA gene sequences were compared with other Theileria spp. available in GenBank by using the BLASTN algorithm. The results indicated that the pathogens from the sampled cattle belonged to the benign T. orientalis group. There were at least three types of Theileria detected: Type A (Chitose), Type B (Ikeda), and Type C (Buffeli) (Table 1). The 18S rRNA gene sequences were submitted to GenBank under accession Nos. KJ020544 to KJ020548.
Based on our PCR analyses, 9 of the 10 blood samples showed amplification with both the 989/990 and the MPSP-F/R primer pairs. The MPSP genes of all PCR-positive samples were cloned and sequenced. Representative sequences were registered in the GenBank under the assigned accession Nos. KJ020544 to KJ020562. When the BLAST analysis was conducted, sample numbers 7 to 9 (KJ020550), 1 to 10 (KJ020551), and 8 to 13 (KJ020557) showed a high identity with the registered database sequence for Theileria Type 5 accession No. AB560825 [5]. Sample numbers 3 to 5 (KJ020552) and 8 to 11 (KJ020556) clustered with the classical benign Type 3 EU584237 isolated in Hubei Province, China; and this type is commonly found on Cheju Island, Korea and on Okinawa Island, Japan. Samples 10 to 22 (KJ020558) clustered with the unclassified T. buffeli accession No. AF236095 from Brisbane, Australia [13].
Moreover, by using the MEGA5 program [14], a neighbor-joining phylogenetic tree was constructed from 32 bovine Theileria MPSP gene sequences, including 14 sequences cloned in this study and 18 sequences from the GenBank database. From the phylogenetic tree, we detected 6 MPSP genotypes that were categorized as MPSP Types 1, 2, 3, 4, and 5 as well as an additional Type 9. The phylogenetic tree indicated that all sequences belonged to 6 of the 9 previously designated types (Fig. 2). T. orientalis Types 6, 7, and 8, which have been reported in China, were not detected in this study [3].
In this study, we detected an additional sequence (KJ020558), which has been classified as Type 9, that was not clustered with the T. orientalis sequences of Types 1 to 8 or N1 to N3 that have been reported in Vietnam [2] (data not shown). Therefore, based on phylogenetic analysis, we describe an additional genotype (Type 9) that is similar to a previously reported MPSP gene that was sequenced in Brisbane, Australia [3]. Based on the phylogenetic tree analysis, many of the sequences from this study are very close to the Australian sequences. Piroplasmosis is not included in the List A or List B of Contagious and Parasitic Diseases for the Animals Imported from Other Countries into the People's Republic of China. By coincidence, the herd sampled in this study was imported from Australia a few months prior to this study. We speculate that the cattle were infected while in Australia. On that basis, it is essential to implement effective quarantine and control measures for vector-borne pathogens in animal populations. Note, in this study, all possible causes of regenerative anemia, such as babesiosis, anaplasmosis, and trypanosomosis were eliminated (data not shown).
Although T. orientalis shows a lower pathogenicity in cattle than Theileria parva and Theileria annulata, occasional disease outbreaks characterized by fever, anemia, anorexia, and reduced growth rate have been reported [91213]. T. orientalis, of which Ikeda (Type 2) is a representative, appears to be closely associated with clinical cases reported in Japan and other Asian countries [412]. Ikeda (Type 2) and Chitose (Type 1) were the predominant types (accounting for 46.4% of the cases) detected in New South Wales, Australia, the epicenter of a theileriosis outbreak [4]. Although Type 2 and Type 1 were detected in this study, they were not the predominant genotypes in our limited dataset. It is common for animals to be infected with mixed genotypes, and that feature presents challenges to the detection and control of theileriosis. After obtaining the diagnostic results, the animals received diminazene aceturate (3 mg/kg of body weight, intramuscularly) every 12 h for 7 days [15]. The therapy resulted in complete recovery of all animals within approximately 10 days; however, additional clinical cases of theileriosis were sporadically reported the following month.
The epidemiology of theileriosis in China has not been described. Basic information about the Theileria spp., such as their life cycles, vectors, modes of transmission, virulences, and host compatibilities require further study. Regardless, the present study demonstrates that infection by T. orientalis is a potentially serious problem in Shaanxi Province, China.
Figures and Tables
Fig. 1
Giemsa-stained blood smear from an infected calf. The arrows point out different polymorphisms of Theileria orientalis. Scale bar = 10 µm.
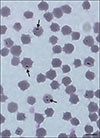
Fig. 2
Phylogenetic relationships among Theileria isolated in Shaanxi Province, China, based on major piroplasm surface protein (MPSP) gene sequences. The bold font indicates sequences obtained from this study. Bootstrap values are shown as percentages at each node based on 1,000 replicates. Branch lengths correlate to the number of substitutions inferred according to the scale shown.

Acknowledgments
This work was supported by the National Beef Cattle Industrial Technology System of China (CARS-38) and the Fund of Jiangsu Agri-animal Husbandry Vocational College (NSF201606). The authors would like to thank the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, for technical help.
References
1. Gubbels MJ, Hong Y, van der Weide M, Qi B, Nijman IJ, Guangyuan L, Jongejan F. Molecular characterisation of the Theileria buffeli/orientalis group. Int J Parasitol. 2000; 30:943–952.
2. He L, Feng HH, Zhang WJ, Zhang QL, Fang R, Wang LX, Tu P, Zhou YQ, Zhao JL, Oosthuizen MC. Occurrence of Theileria and Babesia species in water buffalo (Bubalus babalis, Linnaeus, 1758) in the Hubei province, South China. Vet Parasitol. 2012; 186:490–496.

3. Jeong W, Yoon SH, An DJ, Cho SH, Lee KK, Kim JY. A molecular phylogeny of the benign Theileria parasites based on major piroplasm surface protein (MPSP) gene sequences. Parasitology. 2010; 137:241–249.

4. Kamau J, de Vos AJ, Playford M, Salim B, Kinyanjui P, Sugimoto C. Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasit Vectors. 2011; 4:22.

5. Khukhuu A, Lan DT, Long PT, Ueno A, Li Y, Luo Y, Macedo AC, Matsumoto K, Inokuma H, Kawazu S, Igarashi I, Xuan X, Yokoyama N. Molecular epidemiological survey of Theileria orientalis in Thua Thien Hue province, Vietnam. J Vet Med Sci. 2011; 73:701–705.

6. Kim SJ, Tsuji M, Kubota S, Wei Q, Lee JM, Ishihara C, Onuma M. Sequence analysis of the major piroplasm surface protein gene of benign bovine Theileria parasites in east Asia. Int J Parasitol. 1998; 28:1219–1227.

7. Kubota S, Sugimoto C, Onuma M. A genetic analysis of mixed population in Theileria sergenti stocks and isolates using allele-specific polymerase chain reaction. J Vet Med Sci. 1995; 57:279–282.

8. Liu Q, Zhou YQ, He GS, Oosthuizen MC, Zhou DN, Zhao JL. Molecular phylogenetic studies on Theileria spp. isolates (China) based on small subunit ribosomal RNA gene sequences. Trop Anim Health Prod. 2010; 42:109–114.

9. McFadden AM, Rawdon TG, Meyer J, Makin J, Morley CM, Clough RR, Tham K, Mullner P, Geysen D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. N Z Vet J. 2011; 59:79–85.

10. Ota N, Mizuno D, Kuboki N, Igarashi I, Nakamura Y, Yamashina H, Hanzaike T, Fujii K, Onoe S, Hata H, Kondo S, Matsui S, Koga M, Matsumoto K, Inokuma H, Yokoyama N. Epidemiological survey of Theileria orientalis infection in grazing cattle in the eastern part of Hokkaido, Japan. J Vet Med Sci. 2009; 71:937–944.

11. Rishniw M, Barr SC, Simpson KW, Frongillo MF, Franz M, Dominguez Alpizar JL. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol. 2006; 135:303–314.

12. Shimizu S, Nojiri K, Matsunaga N, Yamane I, Minami T. Reduction in tick numbers (Haemaphysalis longicornis), mortality and incidence of Theileria sergenti infection in field-grazed calves treated with flumethrin pour-on. Vet Parasitol. 2000; 92:129–138.

13. Shiono H, Yagi Y, Thongnoon P, Kurabayashi N, Chikayama Y, Miyazaki S, Nakamura I. Acquired methemoglobinemia in anemic cattle infected with Theileria sergenti. Vet Parasitol. 2001; 102:45–51.

14. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28:2731–2739.

15. Zhang S, Xu Y, Jin Y, Jin Z, Ju Y, Lu C. Development of a Bereriol slow release agent and its preventive effect against Theileriosis sergenti of cattle. Chin J Vet Sci. 1998; 18:367–369.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download