Abstract
The purpose of this study was to evaluate the clinical and imaging characteristics of canine splenic tumors and to establish guidelines for the presurgical assessment of splenic tumors in dogs. Fifty-seven dogs that underwent total splenectomy for the treatment of splenic tumors were evaluated by examining medical records, hematologic results, diagnostic imaging results, and histopathologic results. The maximum lesion size from ultrasonography was significantly different between malignant and benign tumors (p = 0.002). There was a correlation between tumor margination and type of splenic tumors (p = 0.045). Precontrast lesion attenuation on computed tomography was significantly different between splenic malignant and benign tumors (p = 0.001). The mean ± SD precontrast lesion attenuation of malignant tumors was 40.3 ± 5.9 Hounsfield units (HU), and for benign tumors, it was 52.8 ± 6.8 HU. In conclusion, some variables of the imaging examination could be used to distinguish the type of splenic tumor. Based on the study results, using a diagnostic flowchart would be effective in increasing the survival rate of patients with splenic malignant tumors. In addition, fine needle aspiration or magnetic resonance imaging prior to surgical exploration and histopathologic examination may be useful in achieving a more accurate diagnosis.
Splenic tumors commonly observed in older dogs can be life-threatening. Clinical signs in dogs with splenic tumors range from specific signs such as acute collapse with splenic tumor rupture to non-specific signs such as weakness, anorexia, and lethargy [11]. Splenic tumors present a risk of metastasis and spontaneous rupture; therefore, it is important to obtain a quick and accurate assessment before treatment begins [2]. Splenic tumors are usually diagnosed by a combination of hematologic and imaging examinations [17]. In one study on splenic hemangiosarcoma, most dogs showed anemia, thrombocytopenia, and abnormal red blood cell morphology [15].
Ultrasonographic examination is used to evaluate preoperative splenic tumors and the hemoabdomen associated with neoplastic or non-neoplastic diseases before surgery. Non-neoplastic diseases include splenic or liver lobe torsion, trauma, gastric dilatation volvulus, or hepato-biliary disease [10]. Ultrasonography is sensitive to subtle changes or abnormalities in the spleen but is limited in its capacity to assess particular diseases [918]. The accuracy of imaging diagnosis has been greatly improved with the development of computed tomography (CT), which is, currently, widely used in veterinary medicine [7]. However, few studies describing the CT appearance of splenic masses in veterinary medicine have been reported [712].
Long-term prognosis of splenic tumors varies with the histopathologic results and, usually, such results are unknown prior to surgery [10]. Therefore, ultrasound-guided fine needle aspiration (FNA), biopsy, or both can be performed to preclude the need for surgery [39]. Surgeons can predict the prognosis of a patient and determine the appropriate treatment method by obtaining histopathologic results before surgery [8]. In dogs with suspected splenic hemangiosarcoma, clinicians may be hesitant to undertake splenic FNA because of the possibility of seeding tumor cells along the needle tract or causing iatrogenic splenic rupture [16].
The purpose of this study was to evaluate the clinical and imaging characteristics of splenic tumors in dogs and to establish guidelines for presurgical assessment of canine splenic tumors.
Medical records of the Chungnam National University Veterinary Medical Teaching Hospital from 2012 to 2017 were reviewed. Dogs that received total splenectomy were included in the study. Dogs with no histologic examination results, with no presurgical examination results before splenectomy, and that received total splenectomy for reasons other than a splenic mass were excluded from the study. This study was conducted under the guidelines of the Institutional Animal Care and Use Committee at Chungnam National University.
Data obtained from the medical records included clinical data such as signalment and hematologic results (complete blood count, serum biochemical profile). Hematologic results were recorded at least 1 week before surgery. Follow-up data were obtained from medical records or by telephone conversation with the owners. Survival time was defined as the longest follow-up period or time to death after splenectomy.
In this study, ultrasonographic examination was performed with ultrasound equipment (iU22; Phillips, USA). Standard abdominal ultrasonography was performed, and the echotexture of the spleen was analyzed. The following evaluation variables were recorded: maximal size of the tumor (cm), lesion number (solitary, multiple), heterogeneity (heterogeneous, homogeneous), echogenicity (hyperechoic, hypoechoic, isoechoic), and margination (regular, irregular) (Fig. 1).
CT images were obtained by using one of two CT scanners (Alexion [Toshiba, Japan] or Asteion Super [Toshiba]). A standard CT scan technique was used to obtain pre- and postcontrast abdominal CT images under general anesthesia.
Image processing was performed using a commercially available software (Viewrex; Techheim, Korea). The maximal transverse dimension of each tumor was measured by using electronic calipers. The normal splenic parenchyma attenuation value in Hounsfield units (HU) was determined based on the mean of three 20-mm2 regions of interest (ROIs) placed in regions of the parenchyma unaffected by mass lesions. Attenuation of the splenic mass was measured using the maximum circular ROI that could be fit to each mass. The same ROIs were used for pre- and postcontrast images. When there were two or more tumors, the largest tumor was analyzed. When a necrotic lesion was present, mass attenuation was evaluated based on the external areas of the lesion. The evaluation variables of CT images are listed in Table 1.
Statistical analysis was performed by using IBM SPSS software (ver. 24.0; IBM, USA). Independent t-tests were used to investigate significant differences between splenic malignant and benign tumors with respect to dog age. Fisher's exact test was used to examine the relationship between hematologic results and type of splenic tumor. Fisher's exact and linear-by-linear association tests were performed to examine the relationships between ultrasonography variables and splenic tumor types. The Mann-Whitney U test was used to investigate differences between malignant and benign splenic tumors with respect to CT variables and maximal tumor size from ultrasonography. Spearman's correlation coefficient was used to investigate the relationship between splenic malignant tumor size and survival time. Statistical significance was defined as p < 0.05. The Kaplan-Meier product-limit method was used to create survival curves for malignant and benign splenic tumors.
In this study, 57 dogs met the inclusion criteria. Of those, 13 dogs were subsequently excluded. Six dogs that had not undergone histologic examination were excluded, and a further six dogs were excluded because splenectomy was performed for a reason other than the presence of a splenic tumor. Finally, one dog that had no examination results before surgery was excluded. The final study population consisted of 44 dogs. Those 44 dogs underwent total splenectomy for the treatment of splenic tumors between 2012 and 2017. The number of patients, mean age, sex, and mean body weight according to each type of splenic tumor are summarized in Table 2.
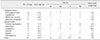
There were 12 dogs with malignant splenic tumors. The mean ± SD age for dogs with malignant tumors was 13.8 ± 4.3 years (median, 14.0 years; range, 3–19 years). There were 32 dogs with benign splenic tumors. The mean ± SD age for dogs with benign tumors was 12.7 ± 2.9 years (median, 13.0 years; range, 4–16 years). There was no significant difference between malignant and benign splenic tumors with respect to age (Fig. 2). The mean ± SD body weight for dogs with malignant splenic tumors was 6.2 ± 2.2 kg (median, 5.9 kg; range, 2.5–9.0 kg) while that for dogs with benign splenic tumors was 8.0 kg ± 7.3 kg (median, 5.7 kg; range, 2.0–35.2 kg). Dogs with malignant tumors, by breed, were Shih-Tzu (n = 4), Maltese (n = 3), Yorkshire terrier (n = 2), Schnauzer (n = 1), and Cocker Spaniel (n = 1). Dogs with benign tumors, by breed, were Shih-Tzu (n = 9), Schnauzer (n = 5), mongrel (n = 3), Maltese (n = 3), Cocker Spaniel (n = 3), Poodle (n = 3), and one or two dogs from 8 other breeds. Hematologic results were obtained for samples from 33 dogs. There were no correlations between hematologic results and the type of splenic tumor (Table 3).
Forty-four dogs had splenic ultrasonography images recorded. The maximum lesion size was significantly different between malignant and benign tumors (p = 0.002). The mean ± SD size of the malignant tumors was 4.1 ± 2.3 cm while that for benign tumors was 1.8 ± 1.7 cm. There was a correlation between margination and type of splenic tumors (p = 0.045). Malignant splenic tumors showed irregular margins in 8 of 12 cases (66.7%), whereas benign tumors had regular margins in 22 of 32 cases (68.8%). The number, echogenicity, and heterogeneity of splenic tumors did not correlate with the type of splenic tumor (Table 4).
Twenty-one dogs had splenic CT images recorded. Precontrast lesion attenuation was significantly different between malignant and benign splenic tumors (p = 0.001). The mean ± SD precontrast lesion attenuation of malignant tumors was 40.3 ± 5.9 HU while for benign tumors it was 52.8 ± 6.8 HU. There were no differences between malignant and benign splenic tumors with respect to other CT variables (Table 5).
Twelve dogs underwent total splenectomies for splenic malignant neoplasia. Excluding one dog that died during surgery, the median survival time of the 11 dogs was 95 days (range, 8–164 days). Of those, the median survival time of splenic hemangiosarcoma dogs (n = 5) was 60 days (range, 8–108 days), and the median survival time of dogs with other splenic malignant tumors was 103 days (range, 42–164 days). Thirty-two dogs with benign tumors underwent total splenectomies. The median survival time of the dogs that underwent splenectomy and were available for follow-up after splenectomy (i.e., 29 dogs) was greater than 25 weeks. Of those dogs, 20 were still alive at the time of final data collection.
The Kaplan-Meier survival curve shows the survival rate for splenic tumors. The two-year survival rate for benign tumors was 77.9%. The 15-week survival rate for malignant tumors, except hemangiosarcoma, was 40%, and 0% for hemangiosarcoma (Fig. 3). There was no correlation between tumor size and survival time (Fig. 4).
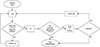
From the ultrasonography images, 95% of malignant splenic tumors were > 2.5 cm in diameter, and 95% of benign tumors were < 2.5 cm. The smallest malignant tumor was 2.0 cm, which can be considered a presurgical assessment criterion. Regarding margin evaluation, malignant splenic tumors had a higher incidence of irregular margins. Based on CT images, precontrast lesion attenuation was less than 47.6 HU in 95% of malignant tumors and greater than 49.1 HU in 95% of benign tumors. The highest precontrast lesion attenuation in malignant tumors was 50 HU, which can be considered a presurgical assessment criterion. Based on the results of this study, a presurgical assessment flowchart for splenic tumor diagnosis in dogs was created (Fig. 5).
In previous studies, the proportion of malignant splenic tumors and splenic hemangiosarcoma followed two-thirds, one-third or fifty-fifty rules [622]. In the present study, the proportion of dogs that underwent splenectomy that had benign splenic tumors (32/44, 72.7%) was notably higher than expected by those ‘rules’. In the present study, the proportion of malignant tumors was 27%, and, of that proportion, the proportion that was hemangiosarcoma was 50%. That outcome is inconsistent with those in other studies reporting on the incidence of benign and malignant splenic tumors in dogs. The difference may be due to bias in the selection of cases. In the present study, cases without histopathologic examination were excluded. In addition, cases with insufficient preoperative examination due to emergency procedures were excluded. Finally, cases were excluded if the owner did not want to provide further treatment for their dog.
There were no correlations between the hematologic results and the type of splenic tumor in this study. Whether hematologic results can be used to diagnose splenic malignant tumors has been a controversial issue [1122]. Patients with anemia and nucleated red blood cells were more likely to have malignant tumors than benign tumors in a study of splenomegaly in dogs [11]. Anemia is potentially life-threatening, as it reduces the amount of oxygen delivered to the tissue. Several studies have reported that dogs with anemia (packed cell volume < 30%) have a lower survival rate than dogs without anemia [1123]. It has also been reported that malignant splenic disease and presence of hemoperitoneum have a negative association with survival time. However, malignancy was not associated with hemoperitoneum [5].
This study did not detect an association between age and splenic tumor type. In a previous study of 539 dogs for splenic masses, a lack of a significant correlation between age and perioperative death was reported [23]. These results suggest that the age of the patient is not a primary consideration for surgical decision making.
Excluding tumor margination results, our results did not reveal correlations between ultrasonography variables and type of splenic tumor. Ultrasonographic images are sometimes inconclusive for the diagnosis of splenic tumors because malignant, and benign splenic tumors may show similar echogenicity patterns while tumors with the same histologic type may show different echo patterns [21]. Accordingly, splenic tumors present with various appearances and that can make it difficult to distinguish the type of splenic tumor by ultrasonography alone [20].
This study showed that the precontrast lesion attenuation of most splenic malignant tumors was lower than 50 HU in CT examinations. This result was similar to that of a previous study, which reported the pre- and postcontrast lesion attenuation levels of most malignant splenic tumors were lower than 55 HU [7].
A previous study in humans showed that magnetic resonance imaging (MRI) is superior to contrast-enhanced CT for the detection and characterization of splenic lesions [13]. In a study of MRI results for focal splenic and hepatic lesions in dogs, the overall accuracy in differentiating malignant from benign tumors was 94.3% (33 of 35 lesions) [4]. These results suggest that the use of MRI can increase the accuracy of splenic tumor diagnosis in dogs.
In this study, dogs with benign splenic tumors survived for a long time after splenectomy. At the time of final data collection, approximately 70% of dogs had survived. Because necropsy was not performed, the exact cause of death was unknown as was the proportion of the deaths that was associated with splenectomy. Side effects of splenectomy are known to include exercise intolerance, insufficient response to reduced cardiac output, decreased response to hypoxia, and susceptibility to erythrocyte parasitism [619]. After splenectomy, the median survival time of dogs with non-neoplastic splenomegaly was reported to be greater than 36 weeks [11]. Dogs with malignant splenic tumors generally have a grave prognosis. At the time of final data collection, all dogs were dead, and the median survival time was 95 days. This result is consistent with those in other studies that reported on the prognosis of splenic hemangiosarcoma in dogs; the median survival time for dogs with splenic hemangiosarcoma treated with surgery alone was reported to be 75 to 86 days [824].
We observed that the Spearman's correlation coefficient between malignant splenic tumor size and survival time was not significant. However, the size of the splenic tumor can be a consideration in surgical decision making. According to the trend observed in this study, a large splenic tumor size is likely to indicate a malignant tumor, and a large tumor size poses a risk of hemorrhage due to spontaneous rupture and the possibility of metastasis. One study demonstrated that dogs with benign splenic masses had higher mass-to-splenic volume ratios and higher splenic weight as a percentage of body weight than those in dogs with hemangiosarcoma [14].
FNA can provide additional data useful in characterizing the type of splenic tumor. In human study, a low complication rate (15/298, 5.0%) and an accurate diagnosis rate (253/298, 84.9%) based on FNA results have been reported [3]. In veterinary medicine, no complications of the aspiration procedure have been reported, but an accurate diagnosis was reported in only 19 of 31 (61.3%) cases [1]. Despite the low complication rate, there are also several disadvantages to FNA. First, successful aspiration depends on the proficiency of the aspirator and the ability of the ultrasonographer. Second, obtaining accurate cytologic interpretations after aspiration may be difficult because obtaining accurate underlying tissue samples may be challenging. Finally, the relative accuracies of cytologic and histologic evaluations of the spleen may vary. For example, in an assessment of 17 malignant splenic tumors, only 8 cytological malignancy results were consistent with the histopathologic results [1]. Furthermore, mis-sampling or incomplete sampling may not distinguish malignancy. In addition, care must be taken when performing the FNA procedure for assessment of cavitary splenic lesions to prevent hemorrhage or seeding.
This study had several limitations. The main limitation of this study is related to its retrospective nature. Histories, clinical signs, and physical examinations were often incompletely described in the medical records. Another limitation of this study is its small sample size. Finally, there was subjective assessment used in obtaining the ultrasonography data. If more objective assessment can be obtained from ultrasonography, more accurate results can be expected.
In conclusion, some variables (i.e., precontrast lesion attenuation, margination type, and maximum lesion dimension) in the diagnostic imaging examination results examined in this study detected significant differences between malignant and benign splenic tumors. Ultrasonography and CT are useful in distinguishing tumor type, and we have included them in a diagnostic flowchart based on the results of this study (Fig. 5). In addition, diagnostic modalities, such as FNA or MRI, may be useful in reaching a more accurate diagnosis before surgical exploration and histopathologic examination, and FNA has been included in this study's flowchart can be effective in providing an early diagnosis of malignant splenic tumors and could increase the survival rate of patients with malignant splenic tumors.
Figures and Tables
Fig. 1
Representative ultrasonography images of splenic tumors. (A) The maximal diameter of the tumor was measured on the longest axis of the mass (bidirectional arrow). (B) The lesion number was one; i.e., a solitary tumor. Heterogeneity was evaluated as homogeneous (C) or heterogeneous (D). Echogenicity was evaluated as hyperechoic (E) or hypoechoic (F). Margination was evaluated as regular (G) or irregular (*) (H).
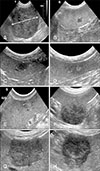
Fig. 2
Box-and-whisker plots of ages in dogs with benign or malignant splenic tumors. The boxes indicate the interquartile range, the horizontal lines within the boxes indicate the median value, and the whiskers indicate the range.

Fig. 3
Kaplan-Meier survival curves for dogs after splenectomy for malignant or benign splenic tumors. The 2-year survival rate for benign tumors was 77.9%, and the 15-week survival rates for other malignant tumors was 40% and was 0% for hemangiosarcoma.

Fig. 4
Relationship between tumor size of malignant splenic tumors and survival time. There was no significant correlation between malignant tumor size and survival time in dogs (p = 0.739).

Fig. 5
Proposed flowchart for presurgical assessment of splenic tumors in dogs. U/S, ultrasonography; CT, computed tomography; HU, Hounsfield units; FNA, fine needle aspiration.
References
1. Ballegeer EA, Forrest LJ, Dickinson RM, Schutten MM, Delaney FA, Young KM. Correlation of ultrasonographic appearance of lesions and cytologic and histologic diagnoses in splenic aspirates from dogs and cats: 32 cases (2002-2005). J Am Vet Med Assoc. 2007; 230:690–696.

2. Brown NO, Patnaik AK, MacEwen EG. Canine hemangiosarcoma: retrospective analysis of 104 cases. J Am Vet Med Assoc. 1985; 186:56–58.
3. Civardi G, Vallisa D, Bertè R, Giorgio A, Filice C, Caremani M, Caturelli E, Pompili M, De Sio I, Buscarini E, Cavanna L. Ultrasound-guided fine needle biopsy of the spleen: high clinical efficacy and low risk in a multicenter Italian study. Am J Hematol. 2001; 67:93–99.

4. Clifford CA, Pretorius ES, Weisse C, Sorenmo KU, Drobatz KJ, Siegelman ES, Solomon JA. Magnetic resonance imaging of focal splenic and hepatic lesions in the dog. J Vet Intern Med. 2004; 18:330–338.

5. Corbin EE, Cavanaugh RP, Schwartz P, Zawadzki KI, Donovan T. Splenomegaly in small-breed dogs: 45 cases (2005-2011). J Am Vet Med Assoc. 2017; 250:1148–1154.
6. Dane DM, Hsia CC, Wu EY, Hogg RT, Hogg DC, Estrera AS, Johnson RL Jr. Splenectomy impairs diffusive oxygen transport in the lung of dogs. J Appl Physiol (1985). 2006; 101:289–297.

7. Fife WD, Samii VF, Drost WT, Mattoon JS, Hoshaw-Woodard S. Comparison between malignant and nonmalignant splenic masses in dogs using contrast-enhanced computed tomography. Vet Radiol Ultrasound. 2004; 45:289–297.

8. Hammond TN, Pesillo-Crosby SA. Prevalence of hemangiosarcoma in anemic dogs with a splenic mass and hemoperitoneum requiring a transfusion: 71 cases (2003-2005). J Am Vet Med Assoc. 2008; 232:553–558.

9. Hanson JA, Papageorges M, Girard E, Menard M, Hebert P. Ultrasonographic appearance of splenic disease in 101 cats. Vet Radiol Ultrasound. 2001; 42:441–445.

10. Ivančić M, Long F, Seiler GS. Contrast harmonic ultrasonography of splenic masses and associated liver nodules in dogs. J Am Vet Med Assoc. 2009; 234:88–94.

11. Johnson KA, Powers BE, Withrow SJ, Sheetz MJ, Curtis CR, Wrigley RH. Splenomegaly in dogs. Predictors of neoplasia and survival after splenectomy. J Vet Intern Med. 1989; 3:160–166.
12. Jones ID, Lamb CR, Drees R, Priestnall SL, Mantis P. Associations between dual-phase computed tomography features and histopathologic diagnoses in 52 dogs with hepatic or splenic masses. Vet Radiol Ultrasound. 2016; 57:144–153.

13. Karakas HM, Demir M, Ozyilmaz F, Cakir B. Primary angiosarcoma of the spleen: in vivo and in vitro MRI findings. Clin Imaging. 2001; 25:192–196.
14. Mallinckrodt MJ, Gottfried SD. Mass-to-splenic volume ratio and splenic weight as a percentage of body weight in dogs with malignant and benign splenic masses: 65 cases (2007-2008). J Am Vet Med Assoc. 2011; 239:1325–1327.

15. Ng CY, Mills JN. Clinical and haematological features of haemangiosarcoma in dogs. Aust Vet J. 1985; 62:1–4.

16. O'Keefe DA, Couto CG. Fine-needle aspiration of the spleen as an aid in the diagnosis of splenomegaly. J Vet Intern Med. 1987; 1:102–109.
17. Patel N, Dawe G, Tung K. Ultrasound-guided percutaneous splenic biopsy using an 18-G core biopsy needle: our experience with 52 cases. Br J Radiol. 2015; 88:20150400.

18. Ramirez S, Douglass JP, Robertson ID. Ultrasonographic features of canine abdominal malignant histiocytosis. Vet Radiol Ultrasound. 2002; 43:167–170.

19. Richardson EF, Brown NO. Hematological and biochemical changes and results of aerobic bacteriological culturing in dogs undergoing splenectomy. J Am Anim Hosp Assoc. 1996; 32:199–210.

20. Schwarz LA, Penninck DG, Gliatto J. Canine splenic myelolipomas. Vet Radiol Ultrasound. 2001; 42:347–348.
21. Solbiati L, Bossi MC, Bellotti E, Ravetto C, Montali G. Focal lesions in the spleen: sonographic patterns and guided biopsy. AJR Am J Roentgenol. 1983; 140:59–65.

22. Spangler WL, Kass PH. Pathologic factors affecting postsplenectomy survival in dogs. J Vet Intern Med. 1997; 11:166–171.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download