1. Furukawa N, Nishioka K, Noguchi T, Kajihara H, Horie K. Port-site metastasis of mucinous borderline ovarian tumor after laparoscopy. Case Rep Oncol. 2014; 7:804–809.
2. Scalici J, Pearsall D, Courtney-Brooks M. Surgical management of uterine carcinosarcoma: is minimally invasive surgery a feasible option? In : Proceedings of the American College of Surgeons (ACS) 2013 Annual Clinical Congress; 2013 Oct 6??0; Washington, D.C. Chicago, IL: American College of Surgeons;2013.
3. Gorai I, Yanagibashi T, Taki A, Udagawa K, Miyagi E, Nakazawa T, Hirahara F, Nagashima Y, Minaguchi H. Uterine carcinosarcoma is derived from a single stem cell: an in vitro study. Int J Cancer. 1997; 72:821–827.
4. Cantrell LA, Blank SV, Duska LR. Uterine carcinosarcoma: a review of the literature. Gynecol Oncol. 2015; 137:581–588.
5. Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol Oncol. 2004; 93:204–208.
6. Felix AS, Stone RA, Bowser R, Chivukula M, Edwards RP, Weissfeld JL, Linkov F. Comparison of survival outcomes between patients with malignant mixed Mullerian tumors and high-grade endometrioid, clear cell, and papillary serous endometrial cancers. Int J Gynecol Cancer. 2011; 21:877–884.
7. Aghajanian C, Sill MW, Secord AA, Powell MA, Steinhoff M. Iniparib plus paclitaxel and carboplatin as initial treatment of advanced or recurrent uterine carcinosarcoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2012; 126:424–427.
8. Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ. Malignant mixed Müllerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. Int J Radiat Oncol Biol Phys. 2004; 58:786–796.
9. Tanner EJ, Leitao MM Jr, Garg K, Chi DS, Sonoda Y, Gardner GJ, Barakat RR, Jewell EL. The role of cytoreductive surgery for newly diagnosed advanced-stage uterine carcinosarcoma. Gynecol Oncol. 2011; 123:548–552.
10. Ahmad G, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2008; CD006583.
11. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Hermans J, Trimbos JB. Complications of laparoscopy: a prospective multicentre observational study. Br J Obstet Gynaecol. 1997; 104:595–600.
12. Karthik S, Augustine AJ, Shibumon MM, Pai MV. Analysis of laparoscopic port site complications: a descriptive study. J Minim Access Surg. 2013; 9:59–64.
13. Abu-Rustum NR, Rhee EH, Chi DS, Sonoda Y, Gemignani M, Barakat RR. Subcutaneous tumor implantation after laparoscopic procedures in women with malignant disease. Obstet Gynecol. 2004; 103:480–487.
14. Zivanovic O, Sonoda Y, Diaz JP, Levine DA, Brown CL, Chi DS, Barakat RR, Abu-Rustum NR. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol Oncol. 2008; 111:431–437.
15. Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003; 91:179–189.
16. Zhang X, Guo X, Zhang A, Wang Y, Zhao J. Influence of carbon dioxide pneumoperitoneum environment on adhesion and metastasis of a human ovarian cancer cell line. Surg Endosc. 2009; 23:108–112.
17. Jacobi CA, Keller H, Mönig S, Said S. Implantation metastasis of unsuspected gallbladder carcinoma after laparoscopy. Surg Endosc. 1995; 9:351–352.
18. Ostrzenski A. Uterine leiomyoma particle growing in an abdominal-wall incision after laparoscopic retrieval. Obstet Gynecol. 1997; 89:853–854.
19. Chan JK, Tian C, Teoh D, Monk BJ, Herzog T, Kapp DS, Bell J. Survival after recurrence in early-stage high-risk epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2010; 116:307–311.

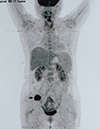
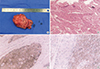




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download