INTRODUCTION
CASE DESCRIPTION
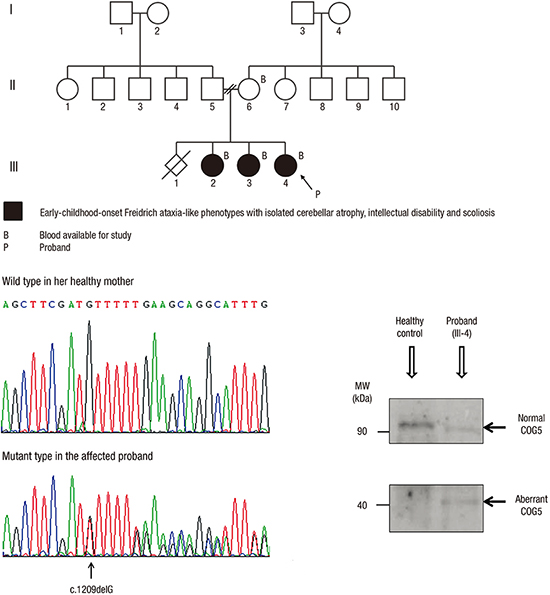
Fig. 1

Table 1
Clinical features and radiologic findings of the patients

Fig. 2

Journal List > J Korean Med Sci > v.32(11) > 1108371



Funding This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Republic of Korea (NRF-2014R1A1A4A01006920).
Author Contributions
Conceptualization: Kim YO.
Data curation: Kim YO, Choi SM, Kim SK, Yoon W, Woo YJ.
Formal analysis: Kim YO, Park C, Hong Y.
Funding acquisition: Kim YO.
Investigation: Kim YO, Yun M, Jeong JH, Park C, Hong Y.
Writing - original draft: Kim YO.
Writing - review & editing: Kim YO, Kim SK, Park C, Hong Y, Woo YJ.
Young Ok Kim 
https://orcid.org/0000-0002-7873-1140
Misun Yun 
https://orcid.org/0000-0001-7818-4442
Jae-Ho Jeong 
https://orcid.org/0000-0002-5544-9572
Seong Min Choi 
https://orcid.org/0000-0003-3138-1881
Seul Kee Kim 
https://orcid.org/0000-0002-1508-5057
Woong Yoon 
https://orcid.org/0000-0002-8598-3127
Chungoo Park 
https://orcid.org/0000-0002-9545-6654
Yeongjin Hong 
https://orcid.org/0000-0002-8115-7362
Young Jong Woo 
https://orcid.org/0000-0003-4717-8394