INTRODUCTION
CASE DESCRIPTION
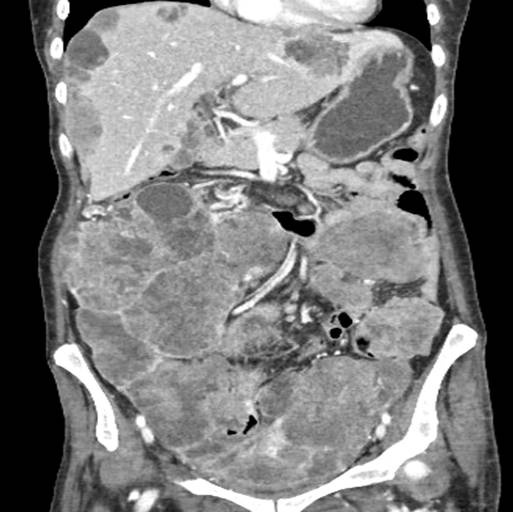
Fig. 1
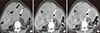
Fig. 2
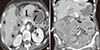
Fig. 3
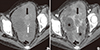
Fig. 4
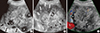
Fig. 5

Journal List > J Korean Med Sci > v.32(12) > 1108345





Hye Won Choi 
https://orcid.org/0000-0003-3735-6791
Hyun Jeong Park 
https://orcid.org/0000-0003-1701-0478
Soon Auk Hong 
https://orcid.org/0000-0002-7902-4608
Sung Bin Park 
https://orcid.org/0000-0002-4155-9260
Eun Sun Lee 
https://orcid.org/0000-0003-0780-7985
Hye Shin Ahn 
https://orcid.org/0000-0001-7260-7467
Jong Beum Lee 
https://orcid.org/0000-0003-0073-7750
Byung Ihn Choi 
https://orcid.org/0000-0002-5613-1881