INTRODUCTION
CASE DESCRIPTION
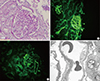
Fig. 1

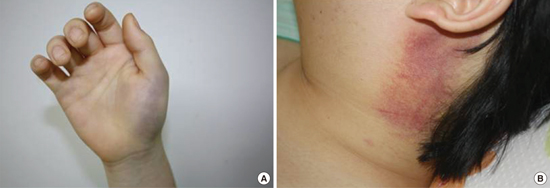
Fig. 2

Fig. 3
Journal List > J Korean Med Sci > v.32(12) > 1108342



Su Woong Jung 
https://orcid.org/0000-0002-9053-924X
Yun Young Choi 
https://orcid.org/0000-0002-6228-6636
In Seung Choi 
https://orcid.org/0000-0002-1139-4485
Seulki Kim 
https://orcid.org/0000-0002-8991-7653
Kyung Hwan Jeong 
https://orcid.org/0000-0001-9265-2468
Ran Song 
https://orcid.org/0000-0003-0104-4091
Sang-Hoon Lee 
https://orcid.org/0000-0003-3655-9546
Hyung-In Yang 
https://orcid.org/0000-0001-9238-6921
Seung-Jae Hong 
https://orcid.org/0000-0002-9803-529X
Yeon-Ah Lee 
https://orcid.org/0000-0001-9961-3947