1. Walsh EE, Falsey AR. A simple and reproducible method for collecting nasal secretions in frail elderly adults, for measurement of virus-specific IgA. J Infect Dis. 1999; 179:1268–1273.
2. Carney AS, Powe DG, Huskisson RS, Jones NS. Atypical nasal challenges in patients with idiopathic rhinitis: more evidence for the existence of allergy in the absence of atopy? Clin Exp Allergy. 2002; 32:1436–1440.
3. Powe DG, Jagger C, Kleinjan A, Carney AS, Jenkins D, Jones NS. ‘Entopy’: localized mucosal allergic disease in the absence of systemic responses for atopy. Clin Exp Allergy. 2003; 33:1374–1379.
4. Rondón C, Doña I, López S, Campo P, Romero JJ, Torres MJ, Mayorga C, Blanca M. Seasonal idiopathic rhinitis with local inflammatory response and specific IgE in absence of systemic response. Allergy. 2008; 63:1352–1358.
5. Fuiano N, Fusilli S, Passalacqua G, Incorvaia C. Allergen-specific immunoglobulin E in the skin and nasal mucosa of symptomatic and asymptomatic children sensitized to aeroallergens. J Investig Allergol Clin Immunol. 2010; 20:425–430.
6. Rondón C, Campo P, Galindo L, Blanca-López N, Cassinello MS, Rodriguez-Bada JL, Torres MJ, Blanca M. Prevalence and clinical relevance of local allergic rhinitis. Allergy. 2012; 67:1282–1288.
7. Blanca-Lopez N, Campo P, Salas M, García Rodríguez C, Palomares F, Blanca M, Canto G, Feo Brito F, Rondon C. Seasonal local allergic rhinitis in areas with high concentrations of grass pollen. J Investig Allergol Clin Immunol. 2016; 26:83–91.
8. Rondón C, Romero JJ, López S, Antúnez C, Martín-Casañez E, Torres MJ, Mayorga C. R-Pena R, Blanca M. Local IgE production and positive nasal provocation test in patients with persistent nonallergic rhinitis. J Allergy Clin Immunol. 2007; 119:899–905.
9. Kim JH, Yoon MG, Seo DH, Kim BS, Ban GY, Ye YM, Shin YS, Park HS. Detection of allergen specific antibodies from nasal secretion of allergic rhinitis patients. Allergy Asthma Immunol Res. 2016; 8:329–337.
10. Campo P, Rondón C, Gould HJ, Barrionuevo E, Gevaert P, Blanca M. Local IgE in non-allergic rhinitis. Clin Exp Allergy. 2015; 45:872–881.
11. Ozcan M, Ataman Gulbudak B, Olcay I, Tekin K, Unal A. Nasal specific IgE for Dermatophagoides in perennial allergic rhinitis and its correlation with serum specific IgE, prick test and nasal provocation test. KBB ve BBC Dergisi. 2007; 15:115–119.
12. Klimek L, Rasp G. Norm values for eosinophil cationic protein in nasal secretions: influence of specimen collection. Clin Exp Allergy. 1999; 29:367–374.
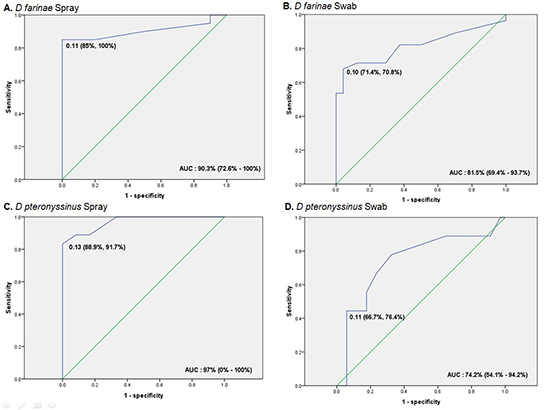
13. Campo P, Rondon C, Prado AP, Salas M, Galindo L, Aranda A, Mayorga C, Ruiz A, Bogas G, Herrero L, et al. A novel method of measuring nasal specific IgE in systemic and local allergic rhinitis patients. J Allergy Clin Immunol. 2016; 137:AB284.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download