1. Abiko T, Sekino H. Syntheses and effect of bursin and it analogs on the reduced B lymphocytes of uremic patients. Biotechnol Ther. 1994; 5:163–170.
2. Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003; 171:4984–4989.

3. Audhya T, Kroon D, Heavner G, Viamontes G, Goldstein G. Tripeptide structure of bursin, a selective B-cell-differentiating hormone of the bursa of fabricius. Science. 1986; 231:997–999.

4. Bhardwaj N. Processing and presentation of antigens by dendritic cells: implications for vaccines. Trends Mol Med. 2001; 7:388–394.

5. Brand A, Gilmour DG, Goldstein G. Lymphocyte-differentiating hormone of bursa of fabricius. Science. 1976; 193:319–321.

6. Cox JC, Coulter AR. Adjuvants: a classification and review of their modes of action. Vaccine. 1997; 15:248–256.

7. Feng X, Liu T, Wang F, Cao R, Zhou B, Zhang Y, Mao X, Chen P, Zhang H. Isolation, antiproliferation on tumor cell and immunomodulatory activity of BSP-I, a novel bursal peptide from chicken humoral immune system. Peptides. 2011; 32:1103–1109.

8. Feng X, Su X, Wang F, Wei J, Wang F, Cao R, Zhou B, Mao X, Zheng Q, Chen P. Isolation and potential immunological characterization of TPSGLVY, a novel bursal septpeptide isolated from the bursa of Fabricius. Peptides. 2010; 31:1562–1568.

9. Feng XL, Liu QT, Cao RB, Zhou B, Ma ZY, Deng WL, Wei JC, Qiu YF, Wang FQ, Gu JY, Wang FJ, Zheng QS, Ishag H, Chen PY. Identification and characterization of novel immunomodulatory bursal-derived pentapeptide-II (BPP-II). J Biol Chem. 2012; 287:3798–3807.

10. Feng XL, Liu QT, Cao RB, Zhou B, Wang FQ, Deng WL, Qiu YF, Zhang Y, Ishag H, Ma ZY, Zheng QS, Chen PY. A bursal pentapeptide (BPP-I), a novel bursal-derived peptide, exhibits antiproliferation of tumor cell and immunomodulator activity. Amino Acids. 2012; 42:2215–2222.

11. Feng XL, Liu QT, Cao RB, Zhou B, Zhang YP, Liu K, Liu XD, Wei JC, Li XF, Chen PY. Characterization and immunomodulatory function comparison of various bursal-derived peptides isolated from the humoral central immune organ. Peptides. 2012; 33:258–264.

12. Ferrara JL. Cytokines and the regulation of tolerance. J Clin Invest. 2000; 105:1043–1044.

13. Jiang W, Jiang P, Li Y, Tang J, Wang X, Ma S. Recombinant adenovirus expressing GP5 and M fusion proteins of porcine reproductive and respiratory syndrome virus induce both humoral and cell-mediated immune responses in mice. Vet Immunol Immunopathol. 2006; 113:169–180.

14. Kreijtz JH, Bodewes R, van Amerongen G, Kuiken T, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine. 2007; 25:612–620.

15. Leenaars M, Koedam MA, Hendriksen CF, Claassen E. Immune responses and side effects of five different oil-based adjuvants in mice. Vet Immunol Immunopathol. 1998; 61:291–304.

16. Li D, Xue M, Wang C, Wang J, Chen P. Bursopentine as a novel immunoadjuvant enhances both humoral and cell-mediated immune responses to inactivated H9N2 Avian Influenza virus in chickens. Clin Vaccine Immunol. 2011; 18:1497–1502.

17. Li DY, Geng ZR, Zhu HF, Wang C, Miao DN, Chen PY. Immunomodulatory activities of a new pentapeptide (Bursopentin) from the chicken bursa of Fabricius. Amino Acids. 2011; 40:505–515.

18. Li DY, Xue MY, Geng ZR, Chen PY. The suppressive effects of bursopentine (BP5) on oxidative stress and NF-κB activation in lipopolysaccharide-activated murine peritoneal macrophages. Cell Physiol Biochem. 2012; 29:9–20.

19. Liu M, Chen H, Luo F, Li P, Pan Q, Xia B, Qi Z, Ho WZ, Zhang XL. Deletion of N-glycosylation sites of hepatitis C virus envelope protein E1 enhances specific cellular and humoral immune responses. Vaccine. 2007; 25:6572–6580.

20. Liu XD, Feng XL, Zhou B, Cao RB, Li XF, Ma ZY, Chen PY. Isolation, modulatory functions on murine B cell development and antigen-specific immune responses of BP11, a novel peptide from the chicken bursa of Fabricius. Peptides. 2012; 35:107–113.

21. Liu XD, Qian Y, Jung YS, Chen PY. Isolation and immunomodulatory activity of bursal peptide, a novel bursal peptide from the chicken bursa of Fabricius. J Vet Sci. 2015; 16:501–507.

22. Liu XD, Zhou B, Cao RB, Feng XL, Li XF, Chen PY. Comparison of immunomodulatory functions of three peptides from the chicken bursa of Fabricius. Regul Pept. 2013; 186:57–61.

23. Liu XD, Zhou B, Feng XL, Cao RB, Chen PY. BP8, a novel peptide from avian immune system, modulates B cell developments. Amino Acids. 2014; 46:2705–2713.

24. Ong WT, Omar AR, Ideris A, Hassan SS. Development of a multiplex real-time PCR assay using SYBR Green 1 chemistry for simultaneous detection and subtyping of H9N2 influenza virus type A. J Virol Methods. 2007; 144:57–64.

25. Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004; 82:488–496.

26. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938; 27:493–497.
27. Rimmelzwaan GF, Baars M, van Beek R, van Amerongen G, Lövgren-Bengtsson K, Claas EC, Osterhaus AD. Induction of protective immunity against influenza virus in a macaque model: comparison of conventional and iscom vaccines. J Gen Virol. 1997; 78:757–765.

28. Roncarolo MG, Levings MK. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol. 2000; 12:676–683.

29. Song Y, Wang X, Zhang H, Tang X, Li M, Yao J, Jin X, Ertl HC, Zhou D. Repeated low-dose influenza virus infection causes severe disease in mice: a model for vaccine evaluation. J Virol. 2015; 89:7841–7851.

30. Steiner JW, Langer B, Schatz DL. The local and systemic effects of Freund’s adjuvant and its fractions. Arch Pathol. 1960; 70:424–434.
31. Wang C, Li X, Wu T, Li D, Niu M, Wang Y, Zhang C, Cheng X, Chen P. Bursin-like peptide (BLP) enhances H9N2 influenza vaccine induced humoral and cell mediated immune responses. Cell Immunol. 2014; 292:57–64.

32. Wang C, Wen WY, Su CX, Ge FF, Dang ZG, Duan XG, Cao RB, Zhou B, Chen PY. Bursin as an adjuvant is a potent enhancer of immune response in mice immunized with the JEV subunit vaccine. Vet Immunol Immunopathol. 2008; 122:265–274.

33. Wen L, Chen SJ, Zhang W, Ma HW, Zhang SQ, Chen L. hsBAFF regulates proliferation and response in cultured CD4
+ T lymphocytes by upregulation of intracellular free Ca
2+ homeostasis. Cytokine. 2011; 53:215–222.

34. Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target antigen for crossreactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985; 82:1785–1789.

35. Zheng QS, Zhang XY, Liu HL, Li P, Chen PY. [The prokaryotic expression and the establishment of the putative indirect ELISA assay for the HA gene for avian influenza virus (AIV) H5N1 subtype]. Wei Sheng Wu Xue Bao. 2005; 45:58–61. Chinese.
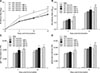


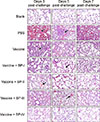







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download