Figures and Tables
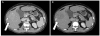 | Fig. 1Liver dynamic CT showed a hypervascular mass (arrows) with a size of 7.5 cm at the fifth hepatic segment. Contrast enhancement in the arterial phase (A) with wash out in the delayed phase (B) was noted. |
 | Fig. 2Liver magnetic resonance image reveals a capsulated mass (arrows) with a size of 7.5 cm. Pre-contrast (A) and arterial phase (B) T1-weighted images show arterial enhancement of the tumor. Delayed phase (C) depicts washout and capsule enhancement. The lesion shows elevated signal on T2-weighted images (D). |
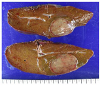 | Fig. 3The specimen contained a round-shaped mass with a size of 5.5 cm, which was well-demarcated around the tissue. |
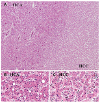 | Fig. 4Pathologic findings show a well-defined mass (A, H&E, ×40). The liver cell cords are one to two cells thick and the cell density is slightly increased compared to the surrounding liver (B, H&E, ×400). There is increased cellularity in the central part of the tumor along with an increased nucleus-to-cytoplasm ratio and irregular nuclear contours (C, H&E, ×400). HCA, Hepatocellucar adenoma; HCC, Hepatocellular carcinoma. |
 | Fig. 5Immunohistochemistry with glutamine synthetase was cytoplasmic weak positive for HCA (A, glutamine synthetase, ×200) and was cytoplasmic strong positive for HCC (B, glutamine synthetase, ×200). For CRP Immunohistochemisty staining, both HCA and HCC showed positive (C, D, CRP, ×200). Evident sinusoidal capillarization with CD34 staining (E, F, CD34, ×200) and membranous pattern for β-catenin staining (G, H, β-catenin, ×200) was noted for both HCA and HCC. HCA, Hepatocellucar adenoma; HCC, Hepatocellular carcinoma. |
References
1. Belghiti J, Cauchy F, Paradis V, Vilgrain V. Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol Hepatol. 2014; 11:737–749.

2. Agrawal S, Agarwal S, Arnason T, Saini S, Belghiti J. Management of hepatocellular adenoma: recent advances. Clin Gastroenterol Hepatol. 2015; 13:1221–1230.

3. Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010; 12:509–522.

4. Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001; 21:877–892.

5. Arif-Tiwari H, Kalb B, Chundru S, et al. MRI of hepatocellular carcinoma: an update of current practices. Diagn Interv Radiol. 2014; 20:209–221.

6. Bioulac-Sage P, Laumonier H, Couchy G, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009; 50:481–489.

7. Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007; 46:740–748.

8. Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011; 60:85–89.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download