Abstract
Esophageal basaloid squamous carcinoma (BSC) is a rare, aggressive variant of squamous cell carcinoma. BSC is usually diagnosed in advanced stage and its prognosis is relatively poor. A 59-year-old male with subepithelial lesion of the esophagus that was incidentally discovered during health promotion examination was referred to our hospital. Esophagogastroduodenoscopy showed a 10-mm bulging mucosa with an intact surface at 34 cm from incisor teeth. Endoscopic ultrasonography revealed a smooth margined homogenous hypoechoic lesion, measuring 11.3×3.9 mm with a submucosal layer of origin. The patient underwent endoscopic mucosal resection of the subepithelial lesion. Pathologic examination of the resected specimen revealed BSC with involvement of vertical margin by tumor. The patient then underwent radiotherapy, and is doing well without recurrence for 35 months. We report a case of esophageal BSC confined to submucosal layer successfully treated with endoscopic resection followed by radiation.
Basaloid squamous carcinoma (BSC) is a rare malignant tumor of the esophagus, and its prevalence is reported to be as high as 3.6% of all esophagectomy cases.1 BSC can occur in the upper aero-digestive tract, thymus, uterine, cervix and anus. Esophageal BSC is most commonly developed in the middle portion of the esophagus, followed by lower and upper portions; males are more frequently affected than females, with the median age of 60s.2 Esophageal BSC, in some cases, can be presented as subepithelial lesion (SEL) of the esophagus.
Esophageal SEL is an unusual tumor accounting for less than 1% of all esophageal tumors.3 Most esophageal SELs are found incidentally due the its lack of symptoms related to SEL. Most SELs of the esophagus are benign tumors, and leiomyoma appears to be the most common type of esophageal SEL. The size of SEL is an important factor in predicting its malignant potential, and in cases of larger size, histologic examination is recommended.4 Here, we report a case of esophageal SEL diagnosed as BSC, which was successfully treated with endoscopic resection and radiation without recurrence for 35 months.
A 59-year-old male was referred to our hospital after incidentally discovering esophageal SEL found on esophagogastroduodenoscopy (EGD) during health promotion examination. He had past medical history of hypertension, history of smoking 6 cigarettes per day for 30 years, and was a social alcohol drinker. Family history included death of his father with laryngeal cancer. EGD showed a 10-mm sized bulging mucosa with intact surface at 34 cm from incisor teeth (Fig. 1). On endoscopic ultrasound (EUS), a smooth margined homogenous hypoechoic lesion — measuring 11.3×3.9 mm in size with a submucosal layer of origin — was observed (Fig. 2). Although esophageal SEL had no malignant features on endoscopic examination, the patient wanted removal of SEL. Endoscopic mucosal resection of esophageal SEL was performed using the snare technique without complication (Fig. 3). On microscopic examination of the resected specimen, 0.6×0.6 cm sized tumor confined to the submucosal layer was diagnosed as BSC with peripheral palisading and central necrosis (Fig. 4A, B); the depth of invasion was 3,000 µm. There was no vascular or lymphatic invasion of the tumor, and the lateral margin was pathologically free of tumor cells. However, the involvement of vertical margin was noted (Fig. 4C). Abdominal and chest computed tomography scan showed no evidence of lymph node or distant metastasis. Positron emission tomography-computed tomography scan showed no evidence of local invasion and distant metastasis. The final diagnosis was esophageal BSC, stage IB by TNM stage. The lesion was defined as 0-Ⅰ (protruding type), according to endoscopic classification based on the guidelines for clinical and pathologic studies of the Japanese Society for Esophageal Disease.5 Due to tumor involvement of resection margin and poor prognosis of esophageal BSC, the patient underwent radiotherapy. Radiation regimen was designed as 50 gray of clinical tumor volume delivered (2 gray per fraction). The follow-up EGD at 35 months from initial treatment showed ulcer scar at 34 cm from incisor teeth (Fig. 5). Pathologic examination of biopsy specimen of esophageal ulcer scar showed mild intraepithelial leukocyte infiltration without evidence of recurrence. The patient is doing well without recurrence for 35 months. To the best of our knowledge, this is the first case of esophageal BSC confined to submucosal layer treated successfully with endoscopic resection combined with radiotherapy.
In our case, the final diagnosis of esophageal SEL was BSC. Esophageal SEL of our case had no EUS features suggestive of malignancy such as large size, irregular margin or ulceration on tumor. BSC is often covered by non-neoplastic stratified squamous epithelium in early stage and present as SEL, making the diagnosis of BSC more difficult using conventional endoscopic forcep biopsy.6 BSC of our case was confided within the submucosal layer and overlying mucosa layer was considered normal. Eckardt and Wassef4 reported that histologic evaluations, including endoscopic mucosal resection, fine needle aspiration, and/or forcep biopsy, are needed in hypoechoic intramural lesion of gastrointestinal tract with size between 1 and 3 cm, dependent on clinical availability and risk. In our case, as esophageal SEL had hypoechoic feature and submucosal layer of origin on EUS and size of SEL was 1.1 cm, endoscopic resection could be one possible option for histologic diagnosis and treatment of esophageal SEL.
In pathologic finding, BSC consisted of small cells resembling basal cells and characterized by nesting, lobular, and trabecular arrangement of solid growth of cells, small crowded cells with scant cytoplasm, hyperchromatic nuclei, and comedo-like necrosis in the center of the base cell nests. The lobules of malignant basaloid cells often display peripheral nuclear palisading, high mitotic activity, and small cystic spaces filled with mucinous material.6
The prognosis of BSC of esophagus has shown conflicting results in previous reports. A previous study of 142 patients with BSC of esophagus treated by surgery and/or radiotherapy or chemotherapy reported that the mean survival time and 5-year overall survival were 32 months and 31.0%, respectively.2 The mean survival time and 5-year overall survival rate in patients with esophageal BSC were lower than those with ordinary squamous cell carcinoma of the esophagus, and the 5-year overall survival rate of patients with stage I BSC was 60.3%.2 However, a review study of approximately 200 patients with BSC of esophagus reported that 2- and 5-year survival rates of BSC were similar to that of patients with ordinary squamous cell carcinoma.6 The prognosis of BSC is poor with poor degree of differentiation and high proliferative activity of tumor and high incidence of metastasis.6 In our case, BSC was confined to submucosa layer and involvement of vertical margin by tumor was noted after endoscopic resection. In previous reports, early tumor stage was associated with favorable survival rate. Nakamura et al.7 reported the possibility of treatment by endoscopic resection for superficial BSC of esophagus based on report that suggested an indifference in the rate of lymph node metastasis between superficial BSC and ordinary squamous cell carcinoma of the esophagus. In our case, positron emission tomography-computed tomography showed no evidence of lymph node or distant metastasis. Due to the aggressive characteristics of BSC and the involvement of resection margin by tumor, radiotherapy was performed to prevent relapse; there was no evidence of recurrence after radiotherapy for 35 months.
Although no standard treatment for esophageal BSC has been established, the primary treatment option for BSC is surgery similar to that for ordinary SCC.2 There was a previous report of esophageal BSC confined to muscularis mucosae treated by endoscopic mucosal resection with cap. In our case, tumor was confined to the submucosal layer of the esophagus.7 Endoscopic resection can be considered as a treatment option in early stage of esophageal BSC without lymph node and distant metastasis; however, eligible criteria for endoscopic resection of esophageal BSC should be further evaluated due to poor prognosis of esophageal BSC. To the best of our knowledge, this is the first case report of esophageal BSC confined to the submucosal layer successfully treated with endoscopic resection and radiation without surgery.
This is a case of esophageal SEL that was diagnosed as BSC confined to the submucosal layer and treated successfully with endoscopic resection and radiation. Further studies are needed to define which patients with esophageal BSC can benefit from endoscopic treatment without surgery.
Figures and Tables
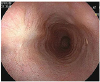
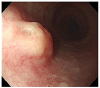 | Fig. 1Esophagogastroduodenoscopy at admission. A 10 mm sized bulging mucosa with an intact surface at 34 cm from incisor teeth was noted. |
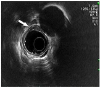 | Fig. 2Endoscopic ultrasonography. A smooth margined homogenous hypoechoic lesion measuring 11.3×3.9 mm in size with a submucosal layer of origin was observed (white arrow). |
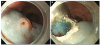 | Fig. 3Lifting of the subepithelial lesion of the esophagus with combination of saline solution, diluted epinephrine, and methylene blue (A), and post endoscopic mucosal resection result (B). |
 | Fig. 4Histologic examination of resected specimen. The tissue showed small crowded cells with scant cytoplasm tumor cells with peripheral palisading (black arrow) (A; H&E, ×40) and central necrosis (black arrow) (B; H&E, ×200) in the submucosal layer of esophagus and involvement of vertical resection margin by tumor was noted (C; H&E, ×100). |
References
1. Cho KJ, Jang JJ, Lee SS, Zo JI. Basaloid squamous carcinoma of the oesophagus: a distinct neoplasm with multipotential differentiation. Histopathology. 2000; 36:331–340.

2. Zhang BH, Cheng GY, Xue Q, et al. Clinical outcomes of basaloid squamous cell carcinoma of the esophagus: a retrospective analysis of 142 cases. Asian Pac J Cancer Prev. 2013; 14:1889–1894.

3. Seremetis MG, Lyons WS, deGuzman VC, Peabody JW Jr. Leiomyomata of the esophagus. An analysis of 838 cases. Cancer. 1976; 38:2166–2177.
4. Eckardt AJ, Wassef W. Diagnosis of subepithelial tumors in the GI tract. Endoscopy, EUS, and histology: bronze, silver, and gold standard? Gastrointest Endosc. 2005; 62:209–212.

5. Japanese Society for Esophageal Disease. Guidelines for clinical and pathologic studies on carcinoma of the esophagus, ninth edition: preface, general principles, part I. Esophagus. 2004; 1:61–88.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download