Abstract
Purpose
Preoperative surgical planning utilizing computed tomography angiography (CTA) has now become a routine in many practices. We analyzed the course of the deep inferior epigastric artery (DIEA) and its perforators (DIEP) that would either facilitate or hinder flap dissection based on CTA to aid surgical planning.
Methods
The 115 consecutive patients who underwent abdominally based free flap breast reconstruction were enrolled in this prospective study. DIEA/P courses were categorized mainly according to their intramuscular courses and their incidences were investigated.
Results
A total of 425 perforators were identified preoperatively on the CTA, with an average number of 3.7 distinctly visualized in the entire flap territory. Eighty-nine perforators (20.9%) had a favorable (less than 1 cm intramuscular course) pattern, namely long submuscular (34.8% of the patients), long subfascial (15.6%), and total circummuscular (13.9%). Overall 56.5% of the patients had at least one favorable DIEA/P. On the other hand, absence of DIEA and absence of adequate (>1 mm) DIEP was reported in 3 and 8 hemiabdomen.
With the advances in the knowledge and technique of the autologous breast reconstruction, maximum safety as well as surgical efficiency is an important issue nowadays12. At present, various imaging modalities are utilized to plan and facilitate the selection and dissection of perforators345. While the deep inferior epigastric artery (DIEA) is known as anatomically consistent, its perforator (DIEP) varies widely between individuals and sides of the abdomen. Specific anatomic variations have been anecdotally reported, and some variation could affect the difficulty or safety of the operation if unrecognized678910.
Preoperative evaluation using computed tomography angiography (CTA) can reveal variants that might affect surgical planning, thereby either encouraging or discouraging perforator dissection. We categorized some patterns of DIEA/P that would either facilitate or hinder dissection, and analyzed their incidences. We also provided detailed information about the CTA protocol used in our institution including multiplanar reconstruction (MPR) imaging and maximum intensity projection (MIP) imaging reconstruction, and delineated their usage.
The following protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2017-1033). All patients who underwent immediate autologous-only breast reconstruction using abdominally based free flap between January 2011 and December 2012 were enrolled in this prospective study. CTA was performed 2 or 3 weeks prior to the surgery unless it was contraindicated for medical reasons or refused by the patient.
All included patients were preoperatively imaged using a 64-multidetector computed tomography (CT) scanner (Somatom Definition 64; Siemens Medical Solutions, Erlangen, Germany) using the following settings: 120 kVp, effective 200 mAs, 0.5 second gantry rotation time, 512×512 matrix, 3 mm slice thickness, 3 mm slice interval on MPR imaging, 20 mm slice thickness, and 5 mm slice interval on MIP imaging. Intravenous nonionic contrasting agent (100 mL of 400 mg iodine/mL iomeprol: Iomeron®; Bracco, Milan, Italy) was injected at a rate of 4 mL/second. The bolus was tracked through the descending aorta starting at the DIEA. Monitoring was started after a 10 seconds delay, and 1 image was taken every 1.25 seconds. If a threshold of 150 Hounsfield units was reached at the descending aorta, CT was performed after another 10 seconds delay. This delay allowed the distal filling of the peripheral small arteries. The raw data were reconstructed in the axial plane at a 0.75 mm slice thickness and 0.4 mm intervals using standard kernels. The reconstructed images were processed at the workstation (PetaVision for Clinics 2; Asan Medical Center, Seoul, Korea).
This anatomic study was mainly based on the 2-dimensional craniocaudal axial images that were scanned in sequence. Perforators that were distinctly visualized (measured diameter >1 mm) on the MPR protocol image were considered as adequate. Perforating vessels piercing the anterior sheath of rectus abdominis muscle from the level of upper margin of the umbilicus to thirty slices (9 cm) below were identified and traced proximally to the point where the main DIEA emerged from the external iliac artery, approximately at the level of the inguinal ligament.
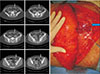
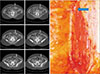
Intramuscular part of the vessel referred to the course truly surrounded by rectus muscle, and excluded such part passing immediately under the anterior sheath or under the deep (posterior) surface of rectus muscle enclosed by fatty perivascular tissue that was readily distinguishable from the muscle belly. Perforators that would facilitate dissection were categorized as ‘favorable’ perforators which had no or very short (<1 cm longitudinal length) intramuscular courses. The circummuscular (septocutaneous) perforator did not have any intramuscular course, and thereby either medially or laterally circumvented the muscle (Fig. 1, 2). The perforators with a long subfascial course or a long submuscular course had a very short intramuscular course (<1 cm based on the longitudinal distance of the muscle, or within three CT slices; Fig. 3, 4).
On the contrary, perforators that would hinder or even rule out dissection were categorized as “unfavorable” perforators. The absence of the DIEA meant there was no pedicle enhanced at the level of the inferior margin of the flap, or there was an apparent severance (Fig. 5). The absence of an adequate DIEP meant that there was no distinct perforator that penetrated into the fascia on the MPR image (Fig. 6). Early muscle penetration referred to a DIEA that entered the rectus abdominis muscle right after it emerged from the external iliac artery without coursing under and/or lateral to the rectus abdominis muscle, and soon arborized into smaller branches, which would result in a long cumbersome intramuscular dissection and unusually small vessel caliber for microanastomosis (Fig. 7).
total of 115 patients were included as our study cohort. No patients had previously received radiotherapy, and 5 patients were ex-smokers who quit smoking at least 4 weeks prior to surgery. No patients were diagnosed with peripheral vasculopathy or diabetes. The mean age of the patients were 42.4 years (range, 28–61 years), and the mean weight of the mastectomy specimens was 350.5 g (range, 100–737 g).
Sixty-seven patients had no abdominal scars. Low transverse scars were found in 24 patients, laparoscopic scars in 14 patients, right lower quadrant (appendectomy) scars in 6 patients, and vertical scars in 8 patients. Four patients had more than one scar.
An average number of 3.7 perforators (range, 0–7 perforators) were distinctly visualized in the entire flap territory on MPR-CTA. Table 1 and 2 demonstrated the incidences of patterns of perforator course that were either favorable or unfavorable. Out of 425 perforators, 89 perforators (20.9%) had a favorable course. Most common were long submuscular course followed by direct vertical muscle penetration (51 perforators, 34.8% of the patients) which was twice more common in the lateral row perforators. Long subfascial course was observed in about 15.6% of the patients which was also more commonly observed in the lateral position. Perforators that did not have any intramuscular course were found in 13.9% of the patients. Overall 56.5% of the patients had at least one favorable DIEA/P course.
The incidences of the ‘absence of DIEA’ and ‘absence of adequate DIEP’ were based on the number of hemiabdomen. Three hemiabdomens in three patients with past surgical history was found to have no DIEA available. There was no adequate perforator (>1 mm on the MPR protocol) in 8 hemiabdomen. One patient had no adequate DIEP on either side of her abdomen. Eleven DIEAs showed the pattern of early muscle penetration: two patients had this pattern of DIEA on both sides of their abdomen. These patients were operated with muscle sparing free transverse rectus abdominis myocutaneous (TRAM) flaps.
All preoperatively selected perforators were of adequate size and demonstrated a visible pulse. Of all the DIEP flaps that were actually performed (n=65), 41.5% were based on the medial perforators and 58.5% were supplied by the lateral perforators.
The full utilization of various image modalities has been shown to be effective with positive clinical benefits, and CTA is one of the most effective and competitive techniques due to its sensitivity, specificity, availability of 3-dimensional reconstruction, and cost11121314. Our preferred CTA protocol (MPR imaging) might be less sensitive than MIP reconstruction that can clearly visualize almost every available perforator. We mark the perforator that is distinctly visible in ≥1 slice with a measured diameter >1 mm when penetrating the fascia in MPR image. The actual diameter may differ from as observed on CTA, but we have always encountered a perforator with an arterial diameter >1 mm having visible pulse when using this selection criterion15. MIP reconstruction is then utilized to confirm the detailed course of the selected perforators.
Katz et al.16 demonstrated a classification scheme. However, only circummuscular pattern was classified as highly favorable, and only obvious absence of the DIEA was classified as hostile. Ireton et al.17 also categorized some patterns of DIEA perforators through systematic review. Our findings have been anecdotally reported in the literature as uncommon variations or case reports6789. The variations were not uncommonly observed in our present series and aided accurate surgical planning and lead to expeditious and safe harvest of the flap.
We categorized perforators mainly according to their intramuscular course which reflected the convenience of dissection. The circummuscular perforator has been reported as an anatomical variration, with various titles such as subfascial, pararectal, and septocutaneous, with an incidence of as high as 15%. If dissection proceeds in two directions, care should be taken in the anterograde dissection because the lateral circummuscular perforator could be mistaken for a laterally escaping branch1819.
Perforators which transpierced the muscle within three CTA slices (<1 cm) were labeled as having a short intramuscular course. Those perforators have two typical patterns, either a long subfascial course or a long submuscular course, and passed the muscle almost vertically. A perforator with a long subfascial course can be useful when preoperatively recognized. If unrecognized, an inadvertent fascial incision may damage this valuable perforator. A perforator that takes a long submuscular course was relatively common, and the most common point of emersion was at the tendinous intersection of the lateral row. It corresponds with the common saying that the lateral perforators usually followed a short linear intramuscular course.
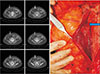
There were courses that discouraged perforator dissection, including DIEA and/or adequate DIEP absence, and very early entry into the muscle and division into smaller branches. DIEA absence is reported as an anatomic variation or surgical sequela69. We experienced 3 patients with unilateral absence or discontinuity of DIEA, all of whom had low transverse abdominal scarring. All 3 patients had previously undergone a hysterectomy prior to 1995. Whilst a Pfannenstiel incision is theoretically and clinically harmless to DIEA, a Maylard incision routinely severs the rectus abdominis muscle and could potentially damage the vessel20.
We encountered 1 patient who did not have an adequate perforator in either side and thus free musclesparing (MS) TRAM was planned and performed. We believe that MS free TRAM with a small piece of muscle is still a safe and reasonable option when adequate DIEP is not identifiable on preoperative CTA. When carefully selected, free DIEP flaps and muscle sparing free TRAM flap resulted in comparable surgical outcome2122.
One inherent limitation of our study is that this anatomic study is mainly based on CTA images. Although we encountered all the pre-identified perforators intraoperatively, the contralateral side of the flap could only be evaluated by the images. Also smaller perforators that were not visualized in our MPR protocol could not be identified nor classified.
In conclusion, preoperative CTA evaluation of DIEA/P can be used to identify favorable as well as unfavorable courses for dissection to aid surgical planning. Overall 56.5% of the patients had at least one favorable DIEA/P course pre-identified by the CTA, most common being long submuscular course followed by long subfascial course.
Figures and Tables
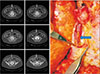 | Fig. 1A medial circummuscular deep inferior epigastric artery perforator. This perforator ran beneath the muscle after medially circumscribing the rectus abdominis, having no intramuscular course (arrows). |
 | Fig. 2A lateral circummuscular deep inferior epigastric artery perforator. This perforator ran on the surface of the muscle before laterally circumventing the rectus abdominis to join the main pedicle (arrows). |
 | Fig. 3Long subfascial course. This perforator ran immediately beneath the fascia (on the surface of the muscle) for a substantial length then pierced the muscle, having a short intramuscular course (arrows). |
 | Fig. 4Long submuscular course. This perforator pierced muscle almost vertically to have a long submuscular course (arrows). |
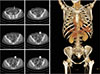 | Fig. 5Absence of left deep inferior epigastric artery. This patient underwent gynecologic surgery in early 1990s (arrow). |
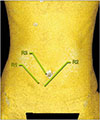 | Fig. 6Absence of sizable perforator in left hemiabdomen. Our multiplanar reconstruction protocol only detected perforators >1 mm in diameter. |
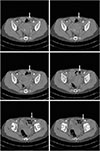 | Fig. 7Early entrance into the rectus abdominis muscle. Left deep inferior epigastric artery penetrated into the muscle almost immediately after branching from the external iliac artery. Note that right deep inferior epigastric artery was still staying at the lateral side of the muscle (arrows). |
Table 1
Favorable perforator courses that facilitated dissection

Circummuscular (septocutaneous) perforator and perforator with long subfascial or submuscular course were classified as favorable perforators. The most common was long submuscular course found in 34.8% of the patients and total circummuscular course was observed in 13.9%. Some patients had multiple favorable perforators.
References
1. Laporta R, Longo B, Sorotos M, Farcomeni A, Amorosi V, Santanelli di Pompeo F. Time-dependent factors in DIEP flap breast reconstruction. Microsurgery. 2017; 37:793–799.

2. Marsh D, Patel NG, Rozen WM, Chowdhry M, Sharma H, Ramakrishnan VV. Three routine free flaps per day in a single operating theatre: principles of a process mapping approach to improving surgical efficiency. Gland Surg. 2016; 5:107–114.
3. Mathes DW, Neligan PC. Preoperative imaging techniques for perforator selection in abdomen-based microsurgical breast reconstruction. Clin Plast Surg. 2010; 37:581–591, xi.

4. Nahabedian MY. Overview of perforator imaging and flap perfusion technologies. Clin Plast Surg. 2011; 38:165–174.

5. Rozen WM, Chubb D, Grinsell D, Ashton MW. Computed tomographic angiography: clinical applications. Clin Plast Surg. 2011; 38:229–239.

6. Chubb D, Rozen WM, Ashton MW. Complete absence of the deep inferior epigastric artery: an increasingly detected anomaly detected with the use of advanced imaging technologies. J Reconstr Microsurg. 2010; 26:209–210.

7. Garusi C, Lohsiriwat V, de Lorenzi F, Manconi A, de Fiori E, Bellomi M. A subfascial variant of the deep inferior epigastric artery demonstrated by preoperative multidetector computed tomographic angiography: a case report. Microsurgery. 2010; 30:156–158.

8. Heo C, Yoo J, Minn K, Kim S. Circummuscular variant of the deep inferior epigastric perforator in breast reconstruction: importance of preoperative multidetector computed tomographic angiography. Aesthetic Plast Surg. 2008; 32:817–819.

9. Rozen WM, Houseman ND, Ashton MW. The absent inferior epigastric artery: a unique anomaly and implications for deep inferior epigastric artery perforator flaps. J Reconstr Microsurg. 2009; 25:289–293.

10. Whitaker IS, Rozen WM, Smit JM, Dimopoulou A, Ashton MW, Acosta R. Peritoneo-cutaneous perforators in deep inferior epigastric perforator flaps: a cadaveric dissection and computed tomographic angiography study. Microsurgery. 2009; 29:124–127.

11. Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg. 2009; 62:1112–1117.

12. Wade RG, Watford J, Wormald JCR, Bramhall RJ, Figus A. Perforator mapping reduces the operative time of DIEP flap breast reconstruction: a systematic review and metaanalysis of preoperative ultrasound, computed tomography and magnetic resonance angiography. J Plast Reconstr Aesthet Surg. 2018; 71:468–477.

13. Fitzgerald O'Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surg. 2016; 5:93–98.
14. Keys KA, Louie O, Said HK, Neligan PC, Mathes DW. Clinical utility of CT angiography in DIEP breast reconstruction. J Plast Reconstr Aesthet Surg. 2013; 66:e61–e65.

15. Kim EK, Kang BS, Hong JP. The distribution of the perforators in the anterolateral thigh and the utility of multidetector row computed tomography angiography in preoperative planning. Ann Plast Surg. 2010; 65:155–160.

16. Katz RD, Manahan MA, Rad AN, Flores JI, Singh NK, Rosson GD. Classification schema for anatomic variations of the inferior epigastric vasculature evaluated by abdominal CT angiograms for breast reconstruction. Microsurgery. 2010; 30:593–602.

17. Ireton JE, Lakhiani C, Saint-Cyr M. Vascular anatomy of the deep inferior epigastric artery perforator flap: a systematic review. Plast Reconstr Surg. 2014; 134:810e–821e.
18. Godfrey PM, Godfrey NV, Romita MC. The “circummuscular” free TRAM pedicle: a trap. Plast Reconstr Surg. 1994; 93:178–180.
19. Hill C, Millar R. Vascular assymetry in a “circummuscular” free TRAM pedicle--a potential hazard. Plast Reconstr Surg. 1997; 99:1199–1200.

20. Bar-Meir ED, Reish RG, Yueh JH, McArdle C, Tobias AM, Lee BT. The Maylard incision: a low transverse incision variant seen in DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg. 2009; 62:e447–e452.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download