Abstract
The temporal branch of the facial nerve is particularly vulnerable to traumatic injuries due to its anatomic location, which often causes severe aesthetic and functional loss in the patient. Moreover, a chronic injury with nerve defect is more difficult to treat compared to acute injury, because it usually needs an additional procedure such as a nerve graft surgical procedure. This case shows a male patient who had a divided temporal branch of the facial nerve one month after an injury. We successfully grafted the split sural nerve and showed a good aesthetic, functional recovery for the patient.
The temporal branch of the facial nerve which innervates corrugator supercilli, frontalis, and orbicularis oculi muscle enables the forehead wrinkling and eyebrow raising in a patient. Therefore, when there is the case of damage to a temporal branch of the facial nerve, this damage subsequently causes an animation deformity noted in the patient's face, which may give rise to considerable aesthetic deficiencies and functional loss of movement in this area1.
The temporal branch of the facial nerve is vulnerable to various traumatic injuries including avulsion, laceration, crushing and penetration. In case of an acute injury without nerve defect, immediate microsurgical neurorrhaphy is possible after nerve stimulator probing to predict the course of the nerve and detect the transected nerve stump2. However, the management of a chronic nerve injury may be more difficult than an acute injury as the result of various factors. Due to the depletion of the neurotransmitter released from the motor end plate within 72 hours from the injury, it is almost impossible to predict the course of nerve using nerve stimulator3. Also, it is difficult to identify the nerve stump, since it is often covered by scar tissue. In a chronic injury, a nerve graft is usually required to relieve problems in this area, because the noted gap distance is too large to achieve a tension-free anastomosis in the patient.
There are no studies noted thus far reporting on the successful nerve graft on the eyebrow level and its long term outcome, nor any report discussing the method of nerve harvest as the preferred procedure for this surgery. We treated a 51-year-old male patient who had a divided temporal branch of the facial nerve by grafting the sural nerve one month after injury. Below, we provide intraoperative findings, as well as the clinical and neurophysiological outcomes, in this case one and half years post operation. Informed consent was obtained from this patient.
A 51-year-old male patient had a laceration located at the left temple area resulting from a fall accident. The patient received a wound closure at an emergency room in a local hospital on the day of accident. One month after the injury, the patient visited our clinic due to inability to wrinkle forehead and raise eyebrow (Fig. 1). The inability to raise left eyebrow resulted in a marked facial asymmetry in the patient, and a physical examination of the patient showed poor contraction tone of the frontalis muscle. Accordingly, an exploration of the wound was planned with the impression of a division of the temporal branch.
We set up a nerve stimulator during surgery, but were not able to confirm frontalis muscle contraction at that time. After that, the predicted course of the nerve was marked based on a review and analysis of the Pitanguy and Ramos4 line. In the meantime, an incision was made around the previous scar and a dissection was performed up to the superficial fascial layer. While it was difficult to identify the transected nerve as it was covered by scar tissue, we were able to find the structure assumed to be divided proximal stump and distal stump. Additionally, a nerve defect at the length of 2 cm was identified, which required a graft using a sural nerve, as the length was not sufficient enough to achieve a tension-free anastomosis. We decided to harvest the sural nerve.
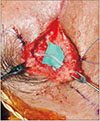
Since the sural nerve is a sensory nerve, a neurorrhaphy was performed to connect the proximal end of the facial nerve to the distal end of the harvested sural nerve, using a 9-0 Nylon with a reverse pattern (Fig. 2). Consequently, the connection of two fascicles is performed by a placement of 9-0 sutures through the interfascicular epineurium. Lastly, we performed a scar revision for a more aesthetic result. There were no surgical complications.
We followed up the patient for 1 year and 6 months postoperatively to review changes after the surgery to the surgical regions. During the period, we evaluated the function of the orbicularis oculi and frontalis muscle by comparing the respective photographs. In what follows (Fig. 3) shows the status of the wound one and half years after the surgery, which shows that the visibility of scar has subsequently been reduced. Likewise, the ability to portray a wrinkle forehead and raise eyebrow on the injured side was improved to a level close to that of the non-injured side (Fig. 4).
At the end of the period, we performed a blink reflex and a facial nerve excitability test for the facial nerve conduction study. For the facial nerve excitability test, we used an electromyography to observe the noted difference in activity of the orbicularis oculi muscles between the injured and non-injured side. In this respect, it was found that the A1 (injured side) and B5 (non-injured side) were set on the middle point between the eyebrow and the hair. The results of the facial nerve excitability test are shown in (Fig. 5). The activity on the injured side was similar to that of the non-injured side. The activities A1 on the injured side were 3.9 Amp mV, while B5, indicating activity on the non-injured side were noted as measuring 3.3 Amp mV (Table 1).
In a blink reflex test, we stimulate the trigeminal nerve in a supraorbital notch, infraorbital area (at the cheek, just below the cheekbone), mental area (between the lip and the chin, 2 cm lateral to the midline), and recorded the potentials and calculated results from these regions. A two-channel recording is performed: R1 and R2. In this case, R1 means that the muscle action potential from the facial nerve (ipsilateral to stimulated side), R2 means reflex response from trigeminal nerve input ipsilateral side and facial nerve output bilaterally. We measured the shortest latency of the R1 and R2 potentials of recordings of each side respectively.
In the blink reflex test of supraorbital, it is noted that the infraorbital trigeminal nerve stimulation, ipsilateral R1 and R2 latency are normal in both side stimulus, and the contralateral R2 is also in the normal range (Table 2, 3). In case of an observation of mental trigeminal nerve stimulation, the ipsilateral R1 latency is not measured, and the ipsilateral R2 latency and contralateral R2 latency are in the normal range (Table 4). In all tests, the R2 latency difference in both sides is in the normal range.
Ultimately, the patient was treated without any neurophysiological abnormality in the facial nerve and blink reflex, whereby displaying satisfactory results of facial symmetry, without an animation deformity.
It is widely accepted that the best functional outcomes are obtainable when the facial nerve is repaired as early as possible after the onset of paralysis56. However, in this case, we performed a sural nerve cable graft on the damaged temporal branch of a facial nerve on a male patient one month after a nerve injury. We evaluated the clinical and neurophysiological findings and verified close to full recovery of the nerve function. Interestingly to note, in this case the recovery process started with tingling sensation after three months, which we deem to be caused by the fact that the nerve gradually grew into the target area, with the nerve graft as a conduit. After half a year postoperatively, some degree of the eyebrow ptosis was observed, but after one year there was a complete recovery.
Evidently, in the nerve graft was there residual myelin debris present at 5 weeks. This was seen in the preexisting fascicles of the graft that did not receive an axonal ingrowth7. The intra-fascicular axons started to cross the distal anastomosis to innervate the distal nerve stump after that. Following a variable period of time (depending on the nature of the injury, location of the injury, and type of repair) the patient in this case observes a “tightening” sensation in the face. Generally, it is noted that the facial tone and symmetry at rest improves significantly between 3 and 6 months post-recovery.
Although the patient has resulted in the satisfactory recovery of motor function, the sense of the posterolateral calf area has subsequently reduced. Immediately after surgery, there was a 50% loss of sensory loss, which recovered up to 70%–80% within the period of months, but there has been no further improvement afterwards. We are currently following up on a long-term basis.
In these terms, considering the incidence of reduced senses in the patient's posterolateral calf region, a further meticulous and minimal harvest is desirable.
This brings us to understand that the average diameter of the sural nerves varied from a minimum of 1.34 mm to a maximum of 2.15 mm, and the maximum average fascicle numbers of 11 to 12 were rapidly attained in the third quarter level which is a most common harvest site8. However, the average diameter of the facial nerve in the middle of the internal acoustic canal is 1.03 mm9. Moreover, it is noted that a branch of facial nerve will be smaller than a facial nerve. Therefore, the sural nerve graft is considerably thicker than the frontal nerve and the 2–3 fascicular repairs are sufficient for the frontal nerve, whereby a split harvest is expected to result in a better outcome in actual surgery in terms of donor site morbidity. The potential for success of a nerve graft is generally optimized when the diameters of the nerve and graft are similar; such as is usually seen when the number and size of the fascicles are similar as well10.
After the flexion of the hip and right knee and dorsiflexion of the right ankle, a short longitudinal incision was made 2 cm lateral to the Achilles tendon at lateral malleolus level. This led to the finding of the nerve pathway, which was located right beside the lesser saphenous vein. Since the sural nerve was thicker than the patient's facial nerve, we decided to split the sural nerve at half the width and 2 cm length, and harvested partially (Fig. 6). Only two fascicles of harvested nerve were used for grafting. The donor site was then repaired and a compression dressing was performed to manage the wound site region against infection.
As we have seen, the facial nerve is typically located in the loose areolar tissue under the superficial musculoaponeurotic system. After a lapse of one month from the time of an injury, the wound will be found as a combination of scar tissues and surrounding tissues, which makes it difficult to differentiate the normal structure of the region. As the nerve stimulator plays a limited role for chronic damages, an accurate anatomical knowledge on the damaged nerve paired with careful dissection may improve the patient surgical outcomes.
Comprehensively taking into account the observations as noted above, the use of an appropriate nerve graft procedure may result in a preferable outcome for patients suffering a chronic nerve damage with a length defect.
Figures and Tables
 | Fig. 1Facial animation of a 51-year-old male patient at one month after injury of the temporal branch of the facial nerve. There was a long scar around the left temple region. The patient was unable to raise the left eyebrow and wrinkle forehead. |
 | Fig. 4The facial animation of the patient with a facial nerve injury at one and half years after the surgery. The patient became capable of raising the left eyebrow to almost the same level as the right one. |
References
1. Seckel BR. Facial danger zones: avoiding nerve injury in facial plastic surgery. Can J Plast Surg. 1994; 2:59–66.

2. Ozmen OA, Falcioni M, Lauda L, Sanna M. Outcomes of facial nerve grafting in 155 cases: predictive value of history and preoperative function. Otol Neurotol. 2011; 32:1341–1346.
3. Kadri PA, Al-Mefty O. The anatomical basis for surgical preservation of temporal muscle. J Neurosurg. 2004; 100:517–522.

4. Pitanguy I, Ramos AS. The frontal branch of the facial nerve: the importance of its variations in face lifting. Plast Reconstr Surg. 1966; 38:352–356.
5. Bascom DA, Schaitkin BM, May M, Klein S. Facial nerve repair: a retrospective review. Facial Plast Surg. 2000; 16:309–313.

6. Terris DJ, Fee WE Jr. Current issues in nerve repair. Arch Otolaryngol Head Neck Surg. 1993; 119:725–731.

7. Spector JG, Lee P, Peterein J, Roufa D. Facial nerve regeneration through autologous nerve grafts: a clinical and experimental study. Laryngoscope. 1991; 101:537–554.
8. Brammer JP, Epker BN. Anatomic-histologic survey of the sural nerve: implications for inferior alveolar nerve grafting. J Oral Maxillofac Surg. 1988; 46:111–117.





 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download