INTRODUCTION
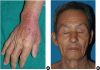
Drug reactions with eosinophilia and systemic symptoms (DRESS) syndrome refers to a potentially life-threatening adverse drug reaction that presents as a skin rash, the presence of eosinophilia in peripheral blood, as well as systemic symptoms caused by fever, enlarged lymph nodes and hepatitis.
12 With a mortality rate of approximately 10%, DRESS syndrome can cause the involvement of internal organs and accordingly, requires accurate and prompt diagnosis and treatment.
1 DRESS syndrome is caused by drugs, such as carbamazepine, allopurinol, phenytoin, phenobarbital, lamotrigine, sulfasalazine and vancomycin, and generally occurs 2-8 weeks after administration of the causative drug.
3
Tuberculosis plays a critical role in healthcare and social issues worldwide. The standard regimen of treatment for tuberculosis involves administration of antituberculosis drugs over a period of 6 months. Antituberculosis drugs are classified as either primary or secondary. Primary drugs are the first-line therapy for tuberculosis as they are highly effective with fewer side effects. Standard primary treatments include isoniazid (INH), ethambutol (EMB), rifampicin (RFP) and pyrazinamide (PZA).
4 Adverse drug reactions caused by antituberculosis drugs increase the probability of treatment failure, mortality and morbidity. As a result, an increased economic burden can occur due to prolonged hospitalization, unscheduled outpatient visits, and additional tests.
5 A few studies have sought to determine the prevalence of severe cutaneous adverse drug reactions (SCAR) caused by antituberculosis drugs. For example, the prevalence of SCAR was reported as approximately 4%–8% in human immunodeficiency virus (HIV)-uninfected patients,
67 but no study has yet confirmed the prevalence of DRESS syndrome, a type of SCAR caused by antituberculosis drugs.
We performed a review of the electronic medical records of all adult patients who were prescribed antituberculosis drugs. Patients were further classified as potential SCAR cases. SCAR cases were retrospectively reconfirmed according to the DRESS syndrome diagnosis criteria
8 suggested by the European Registry of Severe Cutaneous Adverse Reactions to Drugs and Collection of Biological Samples (RegiSCAR) group. Following confirmation and classification by RegiSCAR scoring, the prevalence and clinical characteristics of DRESS syndrome among patients who were administered antituberculosis drugs were evaluated.
MATERIALS AND METHODS
Study design and setting
This study was designed as a retrospective cohort study and involved adult patients (aged ≥ 19 years) with tuberculosis who had been receiving antituberculosis drugs for more than 2 weeks and were followed up more than twice between July 2006 and Jun 2010. We performed a retrospective inspection of the electronic medical records of a university hospital, a tertiary healthcare center that treats nearly 400 patients with tuberculosis annually and employs doctors who specialize tuberculosis.
Inclusion criteria for potential cases
Potential cases were defined as patients who had already been entered into the surveillance system for adverse drug reactions in hospital, who had SCAR related to the administration of antituberculosis drugs, and who met the search requirements used for the search of the electronic medical records. The latter were set to select for patients who required hospitalization for more than 2 days while being on antituberculosis drugs
89 and who requested a consultation from the Department of Dermatology or Allergy due to a generalized skin rash caused by the drugs. Patients also had to meet the following requirements: ≥ 500 eosinophil count in peripheral blood, fever of ≥ 38°C, ≥2 × the upper normal limits of aspartate aminotransferase or alanine aminotransferase, and creatinine levels ≥ 2.0 or a 2-fold increase in the baseline value of creatinine.
Validation of cases with the RegiSCAR scoring system
At first, the evaluation of drug causality was conducted based on the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria by 2 specialists who were familiar with WHO-UMC causality categories.
10 We rechecked the responses to dechallenge or rechallenge procedure with suspected drugs for the causality assessment of DRESS syndrome. All the potential cases of DRESS syndrome caused by antituberculosis drugs were reassessed based on the diagnostic criteria and scoring system suggested by the RegiSCAR group. Scoring was performed in cases with a body temperature ≥ 38°C, enlarged lymph nodes, eosinophil counts in peripheral blood, atypical lymphocytes, skin rash (extent, morphology and biopsy findings), involvement of internal organs (liver, kidney, lung, muscle, heart, pancreas,
etc.), and a symptom duration of ≥ 15 days; serologic studies were performed to exclude other causes, including infection.
8 Patients were classified according to the final score calculated by the scoring system: for example, ≥ 6 was coded as definite, 4–5 as probable, 2–3 as possible and ≤ 1 as no sign of DRESS syndrome.
Clinical and laboratory examinations
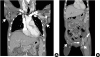
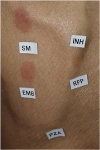
According to the Korean guidelines for tuberculosis, routine follow-up tests conducted during administration of antituberculosis drugs include complete blood counts with differential, routine liver function tests, sputum smear and culture for tuberculosis, and chest X-rays. Such follow-up tests were performed in the first 2 weeks after the beginning of treatment and once per month thereafter. In cases of SCAR that occurred during the administration of antituberculosis drugs and presented as a general skin rash that required hospitalization, additional tests were also conducted. Additional tests included blood culture; serology for A/B/C viral hepatitis; serological test for Epstein-Barr virus (EBV), cytomegalovirus (CMV), herpes simplex virus (HSV) and HIV; sputum culture for bacterial pneumonia; serological test on mycoplasma pneumonia; antinuclear antibodies (ANA); and skin biopsy. In some cases, a chest or abdominal computed tomography (CT) for differentiation the cause of fever was performed. To determine the causative drug, a patch test was conducted with 5 antituberculosis drugs (INH, EMB, RFP, PZA, and streptomycin [SM]) at a 1% dilution during or after tuberculosis treatment. Comorbidities requiring treatment and medicines other than the antituberculosis drugs that were administered to treat such comorbidities were also reviewed.
Outcome measures and assessment
The onset of DRESS syndrome was defined as the start of any clinical symptoms and signs that could indicate DRESS syndrome. Latency was defined as the time from the start of the antituberculosis drug to the onset of DRESS syndrome. The onset of each reaction, including skin reactions, eosinophilia or systemic symptoms, was determined to be related to drug administration. Any drug administrated with the antituberculosis drugs that was previously used by the patient for ≥ 6 months without a cutaneous adverse drug reaction was considered a low-possibility causative drug.
Skin reactions were classified as diffuse infiltrative maculopapules, exfoliation, facial edema, purpura and urticaria. The percentage of total body surface area involved by the skin reaction was also taken into consideration. The first application, median maintenance dose and dosing period of systemic steroids after the onset of DRESS syndrome were also determined. The dosages of systemic steroids were compared in terms of an equivalent dose of prednisolone. Additionally, the rechallenge of antituberculosis drugs for identifying the causative drug, the dose interval between drugs, the additional time needed for tuberculosis treatment due to development of DRESS syndrome and the time spent for dosing were determined.
Statistical analysis
Categorical and dichotomous variables are expressed in absolute numbers and percentages, whereas continuous variables are expressed as mean values and quartiles. The assay to confirm differences among the 3 groups (possible, probable and definite) was performed through RegiSCAR scoring. A Kruskal-Wallis one-way analysis of variance and a χ2 test were used for continuous and categorical variables, respectively. By excluding treatment periods that were < 180 days according to the RegiSCAR score, only the probable and definite groups remained. In these cases, the Mann-Whitney test was used for analysis. The correlation between RegiSCAR scores and continuous variables was determined using Pearson's correlation coefficient obtained from the simple correlation analysis. All significance tests were 2-sided, and P < 0.05 was considered statistically significant.
Ethics statement
This study protocol was approved by the Institutional Review Board of Yonsei University Wonju Severance Christian Hospital (CR318053).
DISCUSSION
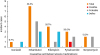
Our retrospective cohort study of adult patients with tuberculosis, the first of its kind, found that the prevalence of DRESS syndrome — one of the SCAR caused by administration of antituberculosis drugs — was 1.2%. EMB was the most common cause of DRESS syndrome, with a prevalence of 53.3% among the first-line antituberculosis drugs; this was followed by RFP (26.7%), PZA (20.0%), SM (13.3%) and INH (6.7%).
There have been a limited number of studies on the prevalence of SCAR requiring the discontinuation of antituberculosis medications. Until now, no study has been conducted to determine the prevalence of DRESS syndrome caused by antituberculosis drugs. Studies conducted on 430 and 156 non-HIV-infected patients receiving antituberculosis drugs in Canada and the UK reported that the prevalences of SCAR were 4% and 8%, respectively.
67 Several demographic differences in the prevalence of SCAR have been found, with a 2.9-fold increase among patients who were ≥ 60 years and a 2.5-fold increase among patients of Asian descent.
6 Asian patients are also more likely to be at risk of DRESS syndrome in cases of allopurinol or carbamazepine.
11 Indeed, in recent studies, the authors suggest a genetic predisposition of DRESS syndrome including antituberculosis drug-associated.
1213 In our study, the prevalence of SCAR caused by antituberculosis drugs and requiring hospitalization was 11.5% (144/1,253), which is higher than the figures previously reported for Western countries.
Recent studies
14 that determined antituberculosis drug-associated DRESS syndrome using the French pharmacovigilance database reported RFP as the most common causative drug. However, the 67 reported potential DRESS syndrome cases in the study included indicators other than tuberculosis. Thirty-six of the patients were treated for tuberculosis, and the most common DRESS syndrome-causing drug among them was INH.
14 Combining their study with previously reported studies, they determined that RFP (70.0%) was the most frequently reported causative drug, followed by INH (65.0%), EMB (58.3%), and PZA (48.4%) in a total of 60 cases.
14 Among the 10 antituberculosis drug-associated DRESS syndrome cases that have been reported in Asia so far,
13151617181920212223 EMB was the most frequently reported drug (7 cases), followed by INH (5 cases), RFP (4 cases), and PZA (3 cases). Six of these cases were associated with a single antituberculosis drug, 1 case was associated with 2 antituberculosis drugs (EMB-RFP), 1 case was associated with 3 antituberculosis drugs (INH-EMB-PZA), and 2 cases were associated with 4 antituberculosis drugs (INH-EMB-RFP-PZA). In our study of 14 patients in whom the causative drug was identified, 10 patients had 1 causative drug, whereas 4 had 2 causative antituberculosis drugs. As the number of causative drugs was limited to 1 or 2 drugs in more than 70% of patients who both maintained the standard treatment of antituberculosis therapy and developed DRESS syndrome due to antituberculosis drugs, the dechallenge and rechallenge of the drugs to identify the causative drug required a proactive approach. This is because discontinuing first-line antituberculosis therapies and replacing them with a second-line regimen can lead to less effectiveness compared to the first-line therapy. The diminished therapeutic effect may cause toxicity issues and the extension of treatment period to up to 2 years.
524 Given the benefits and risks arising from the discontinuation of antituberculosis drugs due to the onset of SCAR, it is recommend that dechallenging and rechallenging also should be carried out during DRESS syndrome.
25 For pulmonary tuberculosis with a maintenance of infectivity in the first 2 weeks of treatment, the discontinuation of the antituberculosis drug may increase the probability not only of the state of infection in the patient but also of the risk of transmitting it to others, which may lead to significant healthcare problems and social issues. When the dosages of antituberculosis drugs are maintained for more than 2 weeks in patients with pulmonary tuberculosis, infectivity is remarkably decreased. Fortunately, DRESS syndrome develops 2–8 weeks after administration of drugs, suggesting it causes decreased infectivity and activity. The common side effects of antituberculosis drugs that require discontinuation of treatment usually develop within 2–8 weeks,
7 whereas DRESS syndrome emerges after these common side effects subside. Determination of the adverse reactions attributable to the dechallenging and rechallenging of drugs is considered beneficial and implies that there is a strong need to actively identify the causative drug. In our study, the median latent period from the administration of the antituberculosis drug(s) to the onset of DRESS syndrome was 42 days, suggesting that the infectivity of tuberculosis may have decreased.
Skin testing can be performed with the suspect drug to determine the causative agent behind cutaneous adverse reactions. This test is generally carried out 6 weeks to 6 months after the improvement of skin lesions when the use of systemic steroids or immunosuppressants has been discontinued for 1 month.
26 For DRESS syndrome or Stevens-Johnson syndrome to prevent recurrence of SCAR, the commercialized form of the drug can be administered at 0.1% of dilution and, subsequently, increased to a concentration of 1%–10% when the test is negative.
26 Previous studies have reported various results depending on the drug used, and there is still a need to conduct further studies on the diagnostic value of patch testing for DRESS syndrome.
27 Therefore, additional studies need to be conducted to propose a standard for patch testing with antituberculosis drugs. If the safety and usefulness of patch testing prior to rechallenging were determined in such additional studies, we would expect that the extension of the tuberculosis treatment course would be avoided by reducing the period of rechallenging.
With the exception of 1 patient, antituberculosis drugs were discontinued in all patients due to suspected DRESS syndrome. The patient who did not discontinue ultimately died, suggesting that the discontinuation of the causative drug is the most important factor in the treatment and prognosis of DRESS syndrome patients.
28 The antituberculosis drugs were discontinued within a median of 18 days after the onset of DRESS syndrome in all but 1 of the 15 patients. Among the 14 patients, 7 showed improvement when sequentially dechallenging the antituberculosis drugs (“sequential dechallenging group”). The remaining 7 patients discontinued all antituberculosis drugs first and then sequentially rechallenged the antituberculosis drugs after their symptoms improved (“sequential rechallenging group”) (
Table 2). Our comparison of the durations of the discontinuation in the sequential dechallenging and sequential rechallenging groups revealed that the median durations were 19 days (IQR 11–57) and 17 days (IQR 11–19) in the dechallenging and rechallenging groups, respectively. These results suggest that the median time of drug discontinuation was shorter in the sequential rechallenging group; however, this difference did not reach statistical significance (
P = 0.405). Additionally, there was no statistically significant difference in the daily dose of steroids (31 mg, IQR 22–98; 33 mg, IQR 22–239, respectively;
P = 0.848), the administration period (14 days, IQR 6–51; 11 days, IQR 7–76, respectively;
P = 0.898), or the duration of tuberculosis treatment (198 days, IQR 145–270; 189 days, IQR 185–277, respectively;
P = 0.848) between the 2 groups. In cases where the causative drug was identified via rechallenging, pruritus and rash developed within 3 days,and eosinophil counts increased to ≥ 1,500/μL within 5 days. The drug-rechallenging sequence differed depending on the patient. Rechallenging was conducted at 3–5-day intervals to determine subsequent symptoms and increases in eosinophil counts. In some other cases of DRESS syndrome, the causative drugs were determined by rechallenging;
141620 however, additional prospective studies are needed to propose standardized methodologies to determine the safety and usefulness of rechallenging, including to ascertain the dosage of the rechallenged drug and the sequence of rechallenging.
DRESS syndrome can affect multiple organ systems, it most frequently affects the lymph nodes; hematologic, hepatic, renal, pulmonary and cardiac manifestations are the next most common.
2 In a separate study, enlarged lymph nodes, reported in 75% of the patients evaluated, was the most common finding in DRESS syndrome.
29 In our study, increased eosinophil counts were observed in all patients, whereas enlarged lymph nodes were found in 77.8% of the patients. Hepatic and renal manifestations followed, with prevalence of 33.3% and 26.6%, respectively. Fever often precedes skin rash,
2 and fever (body temperature ≥ 38°C) developed in 14 of the 15 patients. In some cases, chest or abdominal CT was performed to detect other fever-related febrile disorders. During this procedure, enlarged lymph nodes were often found, albeit inadvertently.
The etiology of DRESS syndrome has not been clearly identified thus far, but it includes the accumulation of reactive drug metabolites caused by abnormalities in the functioning of detoxification enzymes.
30 Given that immunosuppression is often observed at the onset of DRESS syndrome, this information suggests that primary infection (CMV-, EBV- and HHV6-infected) or sequential reactivation is involved in the etiology.
30
It was also observed that high RegiSCAR scores coincided with peak eosinophil counts and short latent periods. Therefore, the probability of DRESS syndrome would be expected to increase when eosinophil counts peak and the suspected clinical presentations emerge early.
There are several limitations to the present study. The results may have limited generalizability as the study was conducted in a single institution with a low prevalence rate. However, the prevalence rate of DRESS syndrome could be identified in our study by analyzing a cohort of adult tuberculosis patients in a tertiary hospital with a high prevalence of patients with tuberculosis (approximately 400 patients per year). Multi-center national studies with larger samples are needed to overcome local bias. This study was designed as a retrospective observational one, which also involves certain limitations. Prospective studies are needed to propose appropriate guidelines for a standardized method to diagnose the causative drug for DRESS syndrome, the standardization of dechalleging and rechallenging, and the steroid dosage.
In conclusion, this study was conducted in a cohort of adult patients with tuberculosis and is the first to identify the prevalence of DRESS syndrome during the course of tuberculosis treatment. DRESS syndrome patients were classified using a scoring system suggested by the RegiSCAR group. Considering the relatively long latent period of DRESS syndrome, it is cautiously suggested that the causative drug can be identified by immediately discontinuing the antituberculosis drugs during the period of low tuberculosis infectivity while maintaining the steroid treatment; in the next step, the antituberculosis drugs could be sequentially rechallenged. Patch testing to determine the safety of each antituberculosis drug before rechallenge may be useful for identifying the causative drug(s).









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download