Abstract
Purpose
Currently, many operations are performed using the single-incision laparoscopic method. Although there have been recent reports on single-incision laparoscopic ileostomy, none have compared this method to conventional laparoscopic ileostomy. This study aimed to assess the safety and feasibility of single-incision laparoscopic ileostomy for anastomotic leakage following laparoscopic low anterior resections.
Methods
From April 2012 to April 2017, 38 patients underwent laparoscopic ileostomy (single-incision; 19 patients referred to as group A, conventional laparoscopy; 19 patients referred to as group B) for anastomotic leakage following laparoscopic low anterior resection. We analyzed surgical and clinical outcomes between the 2 groups. Patients in whom a protective ileostomy was carried out during the initial laparoscopic low anterior resection were excluded from this study.
Results
No significant differences were observed between the 2 groups in terms of patient demographics and initial operation details. Incisional surgical site infections occurred less in group A than in group B (2 of 19 vs. 9 of 19, P = 0.029). The median ileostomy operation time, amount of intraoperative bleeding, parastomal hernia ratio, hospital stay duration after ileostomy, postoperative pain score were not significantly different between the 2 groups.
Surgical techniques are continuously being advanced to improve long-term and short-term surgical outcomes and to improve patients' quality of life. One such advance is the introduction of minimally invasive surgery. These refer to surgical techniques that have the same effects as conventional surgical techniques but with minimal harm on the patients. Laparoscopic surgeries are one of the typical minimally invasive surgeries. Since the first report of laparoscopic ileostomy by Khoo et al. [1], laparoscopy has become popular among surgeons for many types of enterostomies. Compared to the conventional enterostomy, laparoscopic enterostomy is associated with lower postoperative pain, shorter hospital stay, and faster recovery of intestinal functions [23].
Recently, single-incision laparoscopy has been used for several types of abdominal surgeries. Single-incision laparoscopy involves fewer incisions than conventional laparoscopic surgeries, yielding aesthetically superior outcomes as well as benefits of less postoperative pain, lower bleeding, less risk for surgical site infection (SSI), and lower incidence of incisional hernia [4,5,6]. Although there have been several reports of nonscarring single-incision laparoscopic enterostomy [78], none of the previous studies compared it with the conventional laparoscopic enterostomy. In this study, we compared and analyzed single-incision laparoscopic loop ileostomy and conventional laparoscopic loop ileostomy in patients who had developed anastomotic leakage after undergoing laparoscopic low anterior resection.
From April 2012 to April 2017, 38 patients underwent laparoscopic ileostomy (single incision; 19 patients referred to as group A, conventional laparoscopy; 19 patients referred to as group B) for anastomotic leakage following laparoscopic low anterior resection at Incheon St. Mary's Hospital, The Catholic University of Korea. All patients in whom a protective ileostomy was carried out during the initial laparoscopic low anterior resection were excluded from this investigation. Anastomotic leakage was diagnosed based on clinical symptoms such as high fever and abdominal pain, physical examination findings such as an abnormal digital rectal examination result and peritonitis, fecal leakage to the drainage tube, and abdominal computed tomography. Only patients with anastomotic leakage requiring surgery were defined as patients with anastomotic leakage. This study was approved by the Institutional Review Board of Incheon St. Mary's hospital (OC17RESI0107), which also waived the requirement for informed consent due to the retrospective nature of the study. In addition, our study was conducted in accordance with the Helsinki Declaration.
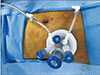
Single-incision laparoscopic loop ileostomy is performed under general anesthesia with the patients in the supine position. The stoma site was preoperatively marked by the ostomy care nurses. A circumlunar incision was made at the ileostomy site and was extended down to the anterior rectus sheath, which was divided in cruciate fashion. The rectus abdominis muscle fibers were then reflected laterally, and the posterior sheath was incised, allowing entry to the peritoneal cavity. This stoma site was then used as a point of laparoscopic access, a single-incision laparoscopic port (Lap single, Sejong Medical, Paju, Korea) was introduced, and pneumoperitoneum was established (Fig. 1). Conventional laparoscopic loop ileostomy was made by a 3-port technique. A 12-mm camera port was placed in the transumbilical site. The 12-mm operative port was placed in the previously selected right-lower-quadrant stoma site and a 5-mm cannula was placed in the suprapubic midline. The cecum was identified, and the ileum was followed by use of a Babcock clamp through the right-lower-quadrant 12-mm ports and a bowel grasper in the 5-mm midline port. After a proper site of the distal ileum was selected that would reach the abdominal wall without tension, the 12-mm cannula was removed. The defect in the anterior rectus sheath created by the 12-mm trocar was extended in a cruciate fashion. Similar to the method described for single-incision laparoscopic ileostomy, entry to the peritoneal cavity was established, and selected ileum was cautiously brought to the abdominal wall. The abdomen was insufflated again, and proximal and distal portions were verified according to anatomic orientation. The remaining cannulas were all removed, and the port sites closed.
All patients in this study received massive laparoscopic saline irrigation to help prevent sepsis and abscess formation. Furthermore, in some patients who showed evidence of macroscopic anastomotic dehiscence on laparoscopic exploration, transanal suturing was attempted to repair the defect.
We reviewed the ileostomy repair operation notes to assess whether parastomal hernias were present or not. For patients who did not have an ileostomy repair, outpatient clinic charts were reviewed to assess if parastomal hernia had existed.
The presence of superficial and deep incisional SSI was investigated. Signs and symptoms associated with the incisional SSI included redness, swelling, warmth, fever, pain, and pus formation.
The data were analyzed using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). Continuous variables, such as age and tumor location were presented as mean ± standard deviation, and operation time was presented as the median number of minutes with the range included for each operation. Categorical variables, such as sex, parastomal hernia development, and SSI ratios were expressed as frequencies. A Student t-test was used to compare continuous variables, whereas categorical variables were compared using the Fisher exact test, as appropriate. A P-value of ≤0.05 was considered significant.
The demographic features of groups A and B are shown in Table 1. No significant differences were observed between the 2 groups in terms of patient age, sex, body mass index, and American Society of Anesthesiologists physical status classification. Moreover, no significant differences were observed between 2 groups regarding the initial operation details (Table 2). Operative and clinical outcomes of both ileostomy procedure types are shown in Table 3. The incisional SSI occurred less in group A than in group B (2 of 19 vs. 9 of 19, P = 0.029). Median ileostomy operation time, intraoperative bleeding, and postoperative discharge day were not significantly different between the 2 groups. Parastomal hernias occurred in 4 patients from group A and 5 patients from group B. The postoperative pain score was similar between the 2 groups.
Remarkable advances have been made in the field of rectal cancer surgery over the past several decades. With increased understanding of mesenteric spread of rectal cancer and the establishment of total mesorectal excision as the standard surgical technique for midlower rectal cancer, local relapse has substantially been reduced. Further, the reduced autonomic nerve injury within the pelvic cavity has maximized the preservation of urinary and sexual functions after surgery [91011]. In addition, advances in surgical techniques and instruments, such as stapler instruments, have increased the proportion of cases in which the anal sphincter is preserved. Nevertheless, complications and mortality after rectal cancer surgery continue to remain as important problems [12]. In particular, anastomotic leakage is the most dangerous and serious complication that occurs in approximately 3%–21% of patients undergoing rectal surgery. It is an important determinant of postoperative mortality, has grave effects such as increasing local relapse and lowering survival rates, and has detrimental effects on long-term prognosis in patients with rectal cancer [13141516].
Rahbari et al. [17] classified anastomotic leakage into grades A, B, and C according to clinical manifestations and reported that protective ileostomy is essential to provide fecal diversion when anastomotic leakage occurs. In particular, for patients with anastomotic leakage who have severe sepsis peritonitis symptoms, or whose abdominal CT reveals wide dispersion of leakage, immediate intra-abdominal irrigation and aggressive surgical treatment such as ileostomy are the standard treatments [1819].
In this study, rate of incisional SSI was less in the single incision laparoscopic ileostomy group than in the conventional laparoscopic ileostomy group. The patients who had anastomotic leakage are more susceptible to SSI. We think that using single port, or reducing number of port incision could reduce the SSI.
We recognize that our study is limited by its retrospective study design and small sample size of 38 patients with short-term follow up at a single institution. Therefore, additional studies in multiple centers with long-term follow-up are required to fully evaluate the safety and feasibility of single-incision laparoscopic ileostomies.
In conclusion, we showed how a single-incision laparoscopic ileostomy can be implemented as a safe and feasible technique following leakage from laparoscopic low anterior resection. Moreover, the procedure can be applied to many other bypass surgical interventions such as those used for cancer obstruction, intestinal perforation and fistulas. In particular, for patients who are at risk for morbidity and mortality, a single-incision laparoscopic ileostomy can offer many advantages as it is a minimally invasive surgical technique [202122].
References
1. Khoo RE, Montrey J, Cohen MM. Laparoscopic loop ileostomy for temporary fecal diversion. Dis Colon Rectum. 1993; 36:966–968.
2. Hollyoak MA, Lumley J, Stitz RW. Laparoscopic stoma formation for faecal diversion. Br J Surg. 1998; 85:226–228.

3. Young CJ, Eyers AA, Solomon MJ. Defunctioning of the anorectum: historical controlled study of laparoscopic vs. open procedures. Dis Colon Rectum. 1998; 41:190–194.
4. Ahmed K, Wang TT, Patel VM, Nagpal K, Clark J, Ali M, et al. The role of single-incision laparoscopic surgery in abdominal and pelvic surgery: a systematic review. Surg Endosc. 2011; 25:378–396.

5. Lim SW, Kim HR, Kim YJ. Single incision laparoscopic colectomy for colorectal cancer: comparison with conventional laparoscopic colectomy. Ann Surg Treat Res. 2014; 87:131–138.

6. Waters JA, Guzman MJ, Fajardo AD, Selzer DJ, Wiebke EA, Robb BW, et al. Single-port laparoscopic right hemicolectomy: a safe alternative to conventional laparoscopy. Dis Colon Rectum. 2010; 53:1467–1472.

7. Zaghiyan KN, Murrell Z, Fleshner PR. Scarless single-incision laparoscopic loop ileostomy: a novel technique. Dis Colon Rectum. 2011; 54:1542–1546.

8. Nguyen HM, Causey MW, Steele SR, Maykel JA. Single-port laparoscopic diverting sigmoid colostomy. Dis Colon Rectum. 2011; 54:1585–1588.

9. Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982; 69:613–616.

10. Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986; 2:996–999.
11. Wibe A, Eriksen MT, Syse A, Myrvold HE, Soreide O. Norwegian Rectal Cancer Group. Total mesorectal excision for rectal cancer--what can be achieved by a national audit? Colorectal Dis. 2003; 5:471–477.
12. Bokey EL, Chapuis PH, Hughes WJ, Koorey SG, Hinder JM, Edwards R. Morbidity, mortality and survival following resection for carcinoma of the rectum at Concord Hospital. Aust N Z J Surg. 1990; 60:253–259.

13. Karanjia ND, Corder AP, Bearn P, Heald RJ. Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg. 1994; 81:1224–1226.

14. Nesbakken A, Nygaard K, Lunde OC. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br J Surg. 2001; 88:400–404.

15. Memon AA, Marks CG. Stapled anastomoses in colorectal surgery: a prospective study. Eur J Surg. 1996; 162:805–810.
16. Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjodahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004; 6:462–469.

17. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010; 147:339–351.

19. Zhu QL, Feng B, Lu AG, Wang ML, Hu WG, Li JW, et al. Laparoscopic low anterior resection for rectal carcinoma: complications and management in 132 consecutive patients. World J Gastroenterol. 2010; 16:4605–4610.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download