INTRODUCTION
Transfusions have saved the lives of many patients experiencing significant blood loss associated with trauma or surgery. Starting in the 1960s, blood transfusions became increasingly cautious, involving component therapy rather than whole blood. In the 1970s and 1980s the level of caution increased due to potential spread of blood-borne infection (e.g., hepatitis and acquired immunodeficiency syndrome) through transfusions. Since 1990, the 30-year-old concept of giving fresh whole blood, instead of packed red cells, has been reemphasized in modern transfusion practices [
12].
After reducing the risk of blood transfusion-related infections through the development of multiple transfusion guidelines [
34], the focus is now on complications associated with the hazard of noninfectious serious hazards of transfusions [
5], transfusion-related immunomodulation and transfusion associated circulatory overload.
Despite current evidence-based transfusion practices, several studies have linked allogeneic blood transfusions with unfavorable outcomes, including increased risks of mortality and various morbidities [
6].
Some of the potential complications associated with allogeneic transfusions can be eliminated or minimized when autologous blood is administered. By 1992, more than 6% of blood transfusions in the United States were autologous [
7]. Acute normovolemic hemodilution (ANH) is an autologous blood transfusion method that involves the removal of whole blood from a patient, while restoring the circulating blood volume with an acellular fluid, shortly before and an anticipated significant blood loss.
A retrospective randomized controlled trial (RCT) by Jarnagin et al. [
8] showed that ANH reduces the requirement for allogeneic red cell transfusions, without altering the complication frequency, in patients undergoing hepatic resection. Another prospective RCT, by Sanders et al. [
9], showed that ANH does not affect the allogeneic transfusion rate during major gastrointestinal surgery. And also Mandai et al. [
10] studied the impact of hemodilution on small-intestinal wound healing in rabbits, and concluded that it does not interfere with small-intestinal wound healing.
However, Fischer et al. [
11] reported that the rate of pancreatic anastomotic complications was higher in patients undergoing ANH than in those receiving usual fluid management. A contradictory human RCT result regarding the impact of ANH on postoperative anastomotic complications has resulted in debate on this issue.
Regardless of the surgery type, surgical site healing is the most important factor in the development of complications or subsequent progression. Surgical site anastomotic leak complications that should be avoided, for the benefit of both the patient and the surgeon because they affect both mortality and morbidity [
12]. Therefore, a number of studies have been conducted to identify the risk factors associated with anastomotic leakage; ANH has not been identified as one.
To further investigate the controversy whether ANH affects postoperative complications, we studied the effects of ANH on gastric anastomosis healing in rats.
METHODS
Animals
This study was approved by the Animal Ethics Committee of Hanyang University (HY-IACUC-18-0006). Humane care was applied in compliance with the “Guide for the Care and Use of Laboratory Animals” (8th edition, National Institutes of Health, USA).
Seven-week-old, male, Sprague-Dawley rats (200 g) were purchased for the study (KOATEC, Pyeongtaek, Korea). The animals were kept in a controlled environment with a 12-hour light/dark cycle and a temperature of 19°–25°; free access to food and water was allowed.
Subject groups
To investigate the causal relationship between ANH and postoperative anastomotic complications, we performed the same gastric surgeries in 16 rats, after a 7-day quarantine and refinement period. The animals were randomly assigned to one of 3 groups, as follows: Group A (ANH, n = 6) underwent ANH followed by gastric surgery. Group N (non-ANH, n = 6) underwent gastric surgery involving using fluid volume management. Group C (control, n = 4) underwent sham operations involving using fluid volume management.
All animals were euthanized on postoperative day 6.
Anesthesia and ANH
For the experiment, rats were placed in a transparent anesthesia induction box supplied with 3 vol % isoflurane in 100% oxygen. When the rats were motionless, they were transferred to a surgical table, with anesthesia maintained using mask-supplied 1.5–2 vol % isoflurane in 100% oxygen under spontaneous breathing.
After placing each animal in a supine position, 24-G angiocatheters (BD angiocath Plus, Becton Dickinson Medical, Singapore) were inserted into the tail vein for fluid management and into the left femoral artery for blood tests and blood removal. The study began when the animal's respiratory pattern and heart rate were stable.
In group A, ANH was performed as follows. The allowable blood volume (V) and estimated blood volume (EBV) of group A were calculated using the following formula.
where EBV is the EBV, Hi is the initial hematocrit, Ht is the target hematocrit, and Hav is the average of the initial and target hematocrits. In our study, the Ht was targeted two-thirds of Hi.
EBV = 64 mL/kg × bodyweight (kg), assuming that the rats had approximately 64 mL of blood/kg of bodyweight.
According to the above formula, 5.8–6.6 mL of blood was withdrawn from left femoral artery in group A. The lost volume was replaced with crystalloid (Plasma Solution-A Injection, CheilJedang Corp., Seoul, Korea) to maintain normovolemia through the tail vein catheter. The common recommendation that was followed was to replace each 1 mL of blood loss with 3 mL of crystalloid. Therefore, each group A animal received 15–18 mL of crystalloid. The duration of the hemodilution process was approximately 15 minutes.
Regardless of the surgical group, total 1 mL of blood was collected from each animal for 2 blood tests, prior to any operation and 5 minutes after completion; this was replenished with 3 mL of crystalloid.
Surgery
The surgeries were performed by 1 surgeon to exclude bias arising from the proficiencies of different surgeons. All of the animals, except group C, underwent gastrectomies that were performed in the same manner. Each surgical site was disinfected with a povidone-iodine solution and a laparotomy was performed using an abdominal midline incision. After the body of stomach was exposed, 2-cm incision was made, and then sutured using a 5-0 union absorbable suture. The skin was closed using a 3-0 nonabsorbable suture, and the operation was terminated. The operative time was approximately 10 minutes.
Six days after the operation, each rat was anesthetized in the same manner as prior to the operation. A midline incision was made via the old incision site and the stomach was excised. The anastomosis site was observed macroscopically and the rat was euthanized.
Histopathologic examination
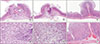
After euthanasia on postoperative day 6, stomach was removed. All stomach specimens were formalin fixed for 24 hours, and all specimen, including anastomosis site were serially sectioned from proximal to distal at approximately 2-mm intervals, and paraffin embedded. All sections were stained with hematoxylin and eosin (H&E) and the entire H&E slides were examined by pathologist, blinded to the experimental manipulation performed, under a light microscope (
Fig. 1).
The grade of inflammatory cells infiltration, neo-angiogenesis, fibroblasts, edema and necrosis were scored as none (0), mild (1), moderate (2), and severe (3) in each case. Formation of inflammatory granulomas and granulation tissue were evaluated by scoring of inflammatory cells infiltration, neo-angiogenesis, fibroblasts and edema, and muscular layer destruction was evaluated by scoring of inflammatory cells infiltration and necrosis.
Statistical analysis
IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA), was used for statistical analyses. The Kruskall-Wallis test was used to detect any statistically significant differences in the histopathological parameters among the groups. If a significant difference was found, a Mann-Whitney U-test was used to identify the between-group differences. P values < 0.05 were accepted as significant.
DISCUSSION
Autologous blood transfusions have been used as an alternative to allogeneic blood transfusions in patients requiring blood transfusions due to the inevitable blood losses that occur during surgery [
13].
Autologous blood transfusions are not only an alternative to the use of blood products that have a limited shelf life, but they are also free from various complications such as the transmission of infections due to the use of allogeneic blood and induced immune responses. This method may be helpful for patients with rare blood types [
7].
Autologous blood transfusions are divided into 4 major categories: preoperative autologous donation (PAD), ANH, intraoperative cell salvage (ICS), and postoperative cell salvage (PCS).
Unlike PAD, ANH does not require surgical schedule adjustments due to preoperative blood collection and does not require the use of expensive equipment, such as the Cell Saver (which is used in ICS and PCS) [
14].
Although ANH is preferred because it is easier and more economical than other transfusion methods, there is no consensus regarding the safety of ANH to date.
Clinically, ANH was introduced about 40 years ago, and has been used as an important means of reducing the use of allogeneic blood. Also ANH has been used for especially in patient who are Jehovah's Witnesses who refuse allogeneic blood transfusions despite the risk of death [
15].
The ANH procedure involves collecting one or more units of blood from the patient, immediately before surgery and after the start of anesthesia; simultaneous infusion of crystalloid or colloid fluids preserves the patient's blood volume.
Using this procedure, fresh whole blood, containing platelets and clotting factors can be prepared, reducing the intraoperative autologous red cell loss due to hemodilution.
ANH has been recognized as a standard care for intraoperative blood preservation by the American Society of Anesthesiologists practice guidelines. A meta-analysis has also demonstrated the modest benefits associated with ANH [
16].
However, ANH should be conducted under the supervision of an experienced anesthesiologist who has gained a good understanding of the patient's circulation during the planning of procedure.
In a randomized trial of patients undergoing ANH to reduce perioperative allogeneic transfusions, ANH resulted in more anastomotic site complications, without reducing allogeneic transfusion, compared with standard patient management [
11]. This contradicted the results of an earlier randomized trial, conducted by the same authors [
8]. The authors concluded that a prospective study would be needed to address the apparent contradiction.
Inspired by a study that later showed that ANH increased transfusion requirements and increased the rate of anastomotic leakage [
11], we investigated the correlation between anastomotic leakage and ANH.
Anastomotic leakage is a serious complication for both the patient and the surgeon, potentially requiring reoperation [
12]. Numerous studies have identified the risk factors of anastomotic leakage as male sex, smoking, obesity, alcohol abuse, preoperative steroid and non-steroidal anti-inflammatory drug use, longer operations, preoperative transfusions, American Society of Anesthesiologists physical status classification III/IV patients, and operative field contamination [
171819]. However, the incidence has not decreased, despite efforts to reduce these risk factors [
20]. Anastomotic leakage is the leading cause of postoperative death, indicating the need to reduce its incidence.
We designed the present study to included ANH as the only independent variable, and we were able to provide fluid supplementation that was exactly calculated. Therefore study biases, such as allogeneic transfusion or fluid overloading, were excluded from this study; such biases could not be controlled in the ANH patients participation in RCT conducted by Fischer et al. [
11].
This experiment, examined the question of whether ANH increases the rate postoperative complications, assuming that ANH is performed well. As shown in
Table 2, there were no differences in the initial hematocrits of the rats in the three groups, but after ANH administration, only group A showed a significant hematocrit decrease. This means between-group differences, which are preconditions of the experimental design proceeded well.
In addition, we excluded the influence of the identified risk factors of anastomotic leakage that are presumed to be the reasons for the conflicting results obtained in the 2 large RCT studies [
811]. Therefore, only the influence of ANH was evaluated, without confounding by other potential anastomotic leakage risk factors.
Both groups A and N showed significant differences from group C, but there was no difference between groups A and N. This means that ANH does not affect healing of the anastomosis and is not, therefore, a risk factor for anastomotic leakage.
One of the limitations of our study was that it was an animal model with limited sample size, and crystalloid was the only fluid expander used. Also, due to ability of rats to resist infection, the risk of anastomosis site infection was lower than in humans. In order to evaluate whether our result of this experiment has enough power, PASS, ver. 14 (NCSS, LLC. Kaysville, UT, USA) was used for statistical analyses. The total sample of 16 subjects achieves 99.9% power to detect differences among the means versus the alternative of equal means using an F-test with a 0.05 significance level. Our use of crystalloid as a supplemental solution eliminated the potential difference between the use of crystalloid and colloids, including in the relative amount of fluid expander required.
In addition, anastomotic leakage is most frequently observed at colorectal sites, but our study involved gastric anastomoses. However, performing the surgeries at the same site in each animal and comparing postoperative healing is more precise in gastric surgeries.
Despite the above limitations exist, this experiment is helpful for determining whether or not ANH is a risk factor for anastomotic leakage. We observed that postoperative complications did not increase in the ANH group, relative to normal surgical management. Future clinical studies investigating the relationship between ANH and wound healing are necessary.
In conclusion, we found that anastomotic complications did not increase following ANH, compared with standard intraoperative management, in gastric anastomosis performed in a rat model.






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download