1. Chen AE, Cantey JB, Carroll KC, Ross T, Speser S, Siberry GK. Discordance between
Staphylococcus aureus nasal colonization and skin infections in children. Pediatr Infect Dis J. 2009; 28:244–246.

2. Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus
. Clin Infect Dis. 2008; 46:Suppl 5. S344–S349.
3. Johansson PJ, Gustafsson EB, Ringberg H. High prevalence of MRSA in household contacts. Scand J Infect Dis. 2007; 39:764–768.

4. Miller M, Cook HA, Furuya EY, Bhat M, Lee MH, Vavagiakis P, et al. Staphylococcus aureus in the community: colonization versus infection. PLoS One. 2009; 4:e6708.
5. Miller LG, Perdreau-Remington F, Bayer AS, Diep B, Tan N, Bharadwa K, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant
Staphylococcus aureus infection from methicillin-susceptible
S. aureus infection: a prospective investigation. Clin Infect Dis. 2007; 44:471–482.

6. Kearns AM, Ganner M, Holmes A. The ‘Oxford Staphylococcus’: a note of caution. J Antimicrob Chemother. 2006; 58:480–481.

7. Strauss R, Amsler K, Jacobs M, Bush K, Noel G. P1321 regional variation in Panton-Valentine leukocidin positivity smong S. aureus isolates in complicated skin and skin structure infections. Int J Antimicrob Agents. 2007; 29:S365–S366.
8. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of
Staphylococcus aureus
. J Clin Microbiol. 2000; 38:1008–1015.

9. Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, et al. Relationships between
Staphylococcus aureus genetic background, virulence factors,
agr groups (alleles), and human disease. Infect Immun. 2002; 70:631–641.

10. Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing
Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999; 29:1128–1132.

11. Cook HA, Furuya EY, Larson E, Vasquez G, Lowy FD. Heterosexual transmission of community-associated methicillin-resistant
Staphylococcus aureus
. Clin Infect Dis. 2007; 44:410–413.

12. Yamamoto T, Takano T, Yabe S, Higuchi W, Iwao Y, Isobe H, et al. Super-sticky familial infections caused by Panton-Valentine leukocidin-positive ST22 community-acquired methicillin-resistant
Staphylococcus aureus in Japan. J Infect Chemother. 2012; 18:187–198.

13. Genestier AL, Michallet MC, Prévost G, Bellot G, Chalabreysse L, Peyrol S, et al.
Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest. 2005; 115:3117–3127.

14. Narita S, Kaneko J, Chiba J, Piémont Y, Jarraud S, Etienne J, et al. Phage conversion of Panton-Valentine leukocidin in
Staphylococcus aureus: molecular analysis of a PVL-converting phage, phiSLT. Gene. 2001; 268:195–206.

15. Laifer G, Frei R, Adler H, Fluckiger U. Necrotising pneumonia complicating a nasal furuncle. Lancet. 2006; 367:1628.

16. Couvé-Deacon E, Tristan A, Pestourie N, Faure C, Doffoel-Hantz V, Garnier F, et al. Outbreak of Panton-Valentine leukocidin-associated methicillin-susceptible
Staphylococcus aureus infection in a rugby team, France, 2010–2011. Emerg Infect Dis. 2016; 22:96–99.

17. Tinelli M, Monaco M, Vimercati M, Ceraminiello A, Pantosti A. Methicillin-susceptible
Staphylococcus aureus in skin and soft tissue infections, Northern Italy. Emerg Infect Dis. 2009; 15:250–257.

18. Knox J, Uhlemann AC, Miller M, Hafer C, Vasquez G, Vavagiakis P, et al. Environmental contamination as a risk factor for intra-household Staphylococcus aureus transmission. PLoS One. 2012; 7:e49900.
19. Uhlemann AC, Knox J, Miller M, Hafer C, Vasquez G, Ryan M, et al. The environment as an unrecognized reservoir for community-associated methicillin resistant Staphylococcus aureus USA300: a case-control study. PLoS One. 2011; 6:e22407.
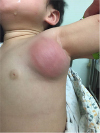
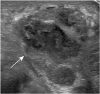




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download