This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Cow's milk protein causes an unfavorable and unwanted reaction in some individuals called cow's milk protein allergy (CMPA). It is more often imprecise and easily missed in primary care settings. Cow's Milk-related Symptom Score (CoMiSS) was developed as a screening and awareness tool to suggest the presence of CMPA using general, dermatological, gastrointestinal, and respiratory symptoms.
Objective
Assess the utility of CoMiSS in the diagnosis of CMPA in Indian children aged between 0 and 24 months.
Methods
A pilot multicentric, observational, longitudinal study was conducted over a period of 4 months among infants aged 0–24 months with symptoms suggestive of CMPA to measure the positive and negative predictive value of CoMiSS. A predesigned questionnaire was used to record the information via CoMiSS. The patients were confirmed of having CMPA via oral food challenge/skin prick test or ImmunoCAP test.
Results
A total of 83 children were enrolled in the study and majority of them had gastrointestinal complaints (61%, 51 of 83) followed by respiratory (41%, 34 of 83) and skin complaints (33%, 27 of 83). CoMiSS was >12 in 72.3% of the infants and amongst them 84.3% were confirmed via oral food challenge/ImmunoCAP test. The positive and negative predictive values for CoMiSS were 93% and 33% respectively.
Conclusion
CoMiSS can help predict CMPA in children aged less than 2 years in the Indian primary care setting, aiding in early diagnosis. Prospective randomized studies are needed to evaluate the use of CoMiSS further.
Keywords: Cow's milk protein allergy, Cow's Milk-related Symptom Score, ImmunoCAP test, Child, Allergy
INTRODUCTION
Infancy is a period of rapid growth and development and during this critical period, improper immunefunctioning, along with other contributing factors, may increase the risk of allergies—primarily food allergies [
1]. Food allergy is as an immunologically mediated hypersensitivity reaction to any food, including immunoglobulin E (IgE)-mediated and/or non-IgE-mediated allergic reactions. Generally, food allergies cause relatively mild and minor symptoms, however some of them can cause severe or grave reactions [
23]. Cow's milk protein allergy (CMPA) remains the most prevalent food allergy among infants and young children. CMPA is a reproducible adverse reaction of an immunological nature induced by cow's milk proteins [
4]. The global prevalence of CMPA is about 2%–7.5%; however, not enough data are available in India to determine its local prevalence. While it is an established disease in the developed world, the steady rise in the prevalence of CMPA in developing countries cannot be overlooked [
56].
Most symptoms of CMPA involve the skin (e.g., atopic dermatitis); the gastrointestinal tract (e.g., vomiting, diarrhea, constipation); and the airways (e.g., wheezing, sneezing)—or, they are more general, such as colic. However, none of these symptoms are specific or definitive in nature. This may lead to over diagnosis, underdiagnoses, or misdiagnosis of the disease, causing significant anguish among parents and infants. CMPA is most often confused with lactose intolerance, infantile colic, gastroesophageal reflux disease (GERD), or functional gastrointestinal disorders—leading to delayed diagnosis, repeated consultations, and inaccurate management. In addition, considering that CMPA has remained a topic of debate and controversy as far as diagnosis is concerned, and considering the lack of definitive guidelines, it remains a substantial burden from the clinical standpoint. Thus, as CMPA is easily missed, making a correct and early diagnosis while minimizing the burden on the patient and family remains a challenge [
47].
Primary care physicians are the first to encounter cases of CMPA. Inadequate awareness among of CMPA can predominantly attributed to the lack of guidelines and unswerving diagnostic methods. Hence, there is a dire need for a tool such as Cow's Milk-related Symptom Score (CoMiSS) that brings about awareness to help recognize CMPA in infants. CoMiSS was designed to facilitate and assess symptoms from different organ systems associated with CMPA among general physicians. CoMiSS includes parameters such as general, dermatological, gastrointestinal, and respiratory symptoms and takes anywhere between 5 and 15 minutes to complete [
8]. CoMiSS is suggested to help evaluate and quantify symptoms of CMPA, facilitate efficient decision-making and support right management. However, it cannot be overemphasized that this symptom scoring tool doesn't replace food challenge test and cannot be considered as a diagnostic tool [
89].
Although many infants with high CoMiSS have no impaired growth or weight gain, faltering of these parameters may suggest an organic disease, of which CMPA is a possible cause.
In this purview, an observational study was conducted to assess the utility of CoMiSS in the diagnosis of CMPA in Indian children aged between 0 and 24 months.
MATERIALS AND METHODS
The study was a pilot, multicentric, cross-sectional, observational study conducted with 5 pediatricians in their respective centers throughout India over a period of 6 months (July–December 2016) in children aged 0–24 months. The Department of Pediatrics, Institute of Medical Sciences, and Banaras Hindu University were the leading centers for observation. The study was approved by the ethics committee. Infant and children presenting with one or more for the following CMPA symptoms were included in the study: cutaneous (atopic dermatitis and urticaria), respiratory (cough and dyspnea, rhinitis), and gastrointestinal (digestiveregurgitation, vomiting, rectal bleeding, constipation and diarrhea). Presence of one or more gastrointestinal symptoms, or an association of these symptoms with skin or respiratory symptoms, was also included as a part of the study. Children with acute diarrhea, congenital abnormalities, and established causes of other pediatric illness were excluded from the study. The study was carried out with the intention of treating the child.
A predesigned questionnaire, with informed consent obtained from any one parent/primary care giver of the child, was used to collect information pertaining to the general characteristics and anthropometry, medical and feeding history and clinical examination via CoMiSS.
Confirmatory diagnosis of CMPA was done via oral food challenge, skin prick test (SPT), or ImmunoCAP test. Oral food challenge test was carried out 15 days after an elimination diet and absence of symptoms. A follow-up period of up to 4 weeks was done after the test.
The food challenge test included following a diet free of cow's milk and its derivatives for at least 2 weeks, using soy protein isolate formula among infants as well as nursing mothers. Irrespective of symptom remission the children were subjected to an open oral food challenge test using the following protocol (
Fig. 1):
 | Fig. 1 Schematic flow of study.
|
(1) At time 0: About 2 mL of cow's milk was administered to the skin of the left forearm.
(2) At time 15: About 2 mL of cow's milk was administered to the perioral region.
(3) From the time 30: Every 15 minutes, cow's milk was gradually given in portions of 1, 4, 10, 20, 20, 20, and 25% of the total volume calculated (0.5 g of cow's milk protein without lactose/kg) until symptom onset.
The asymptomatic children were considered negative for CMPA and in children whom the symptoms reappeared were considered positive for CMPA. The data thus collected were coded and analyzed (via. SPSS trial ver. 16.0 [SPSS Inc., Chicago, IL, USA]), and appropriate statistical tests were applied to draw relevant conclusions. The statistical tests were significant, at p ≤ 0.05.
RESULTS
Baseline characteristics
A total of 83 children with suggestive symptoms of CMPA were included in the study. Mean age and birth weight were12.3 ± 6.4 months and 2.9 ± 0.4 kg respectively. The current mean weight was 8.6 ± 2.7 kg, and the present height was 72 ± 9.1 cm. The mean gestational age was 38.7 ± 1.9 months (
Table 1).
Table 1
Patient characteristics (n = 83)

|
Characteristic |
Value |
|
Age (mo) (n = 83) |
12.5 ± 6.4 |
|
Birth weight (kg) (n = 77) |
2.9 ± 0.4 |
|
Present weight (kg) (n = 79) |
8.6 ± 2.7 |
|
Crown heel length (n = 74) |
72.0 ± 9.1 |
|
Gestational age (mo) (n = 71) |
38.7 ± 1.9 |
|
Sex |
|
|
Male |
52 (62.7) |
|
Female |
31 (37.3) |
|
Developmental milestones as per age |
|
|
Achieved |
82 (98.8) |
|
Not achieved |
1 (1.2) |
|
Immunization as per age completed |
74 (89.2) |
|
Exclusively breastfed for the first 6 mo (if child is aged>6 mo) |
26 (31.3) |
|
Age of introduction of complementary food/cow's milk |
|
|
Before 6 mo |
55 (66.3) |
|
At 6 mo |
21 (25.3) |
|
After 6 mo |
7 (8.4) |
|
Chief complaint |
|
|
Gastrointestinal complaints |
51 (61.4) |
|
Skin complaints |
27 (32.5) |
|
Respiratory complaints |
34 (41.0) |
|
Family history of any allergic condition |
|
|
Asthma |
11 (13.3) |
|
Eczema |
11 (13.3) |
|
Rhinitis |
9 (10.8) |
|
Urticaria/angioedema (hives/swelling) |
4 (4.8) |
|
Medication allergies |
2 (2.4) |
|
Food allergies |
2 (2.4) |
|
None |
49 (59.0) |
|
Presence of allergic conditions |
|
|
Asthma |
8 (9.6) |
|
Eczema |
18 (21.7) |
|
Rhinitis |
25 (30.1) |
|
Urticaria/angioedema (hives/swelling) |
18 (21.7) |
|
Medication allergies |
2 (2.4) |
|
Food allergies |
4 (4.8) |
|
None |
40 (48.2) |
|
Child on any medication, yes |
44 (53.0) |
|
Symptoms in relation to a food/cow milk ingestion*
|
|
|
Gastrointestinal |
41 (49.4) |
|
Skin |
50 (60.2) |
|
Respiratory |
64 (77.1) |
|
None |
1 (1.2) |
|
The child is a suspected case of |
|
|
Cow's milk allergy |
74 (89.2) |
|
Food allergy other than cow milk allergy |
13 (15.7) |
|
Food intolerances |
20 (24.1) |
|
Other allergies |
9 (10.8) |

Sixty-three percent (52 of 83) of the infants were male, and 37% (31 of 83) were female. Almost all the infants achieved their age-specific developmental milestones. Eighty-nine percent (74 of 83) of the infants were on track with their immunization schedule. Thirty-one percent (26 of 83) of the children were exclusively breastfed or had been exclusively breastfed up to the age of 6 months. Twenty-five percent (21 of 83) of children had timely initiation of complementary feeding, whereas 66% (55 of 83) were introduced complementary feeding before 6 months.
Most infants were brought to the clinic due to gastrointestinal complaints (61%, 51 of 83), followed by respiratory (41%, 34 of 83) and skin complaints (33%, 27 of 83). About 41% (34 of 83) of the infants had a positive family history of allergies such as asthma, eczema, rhinitis, urticaria, angioedema, and other medical or food allergies. Gastrointestinal symptoms were seen in 41 infants, skin complaints were seen in 50 infants, and respiratory symptoms were seen in 64 infants. Almost 89% (74 of 83) of the children were suspected to have CMPA, based on their clinical presentation (
Table 1).
Cow's Milk-related Symptom Score
The mean CoMiSS of the children was 16.2 ± 6.8. The minimum CoMiSS was 2, and the maximum was 32. A score of above 12 was seen in 72% (60 of 83) of the children, warranting further evaluation and need for confirmatory diagnosis of CMPA. Overall, 84.3% (70 of 83) of the children were diagnosed with CMPA via oral food challenge/ImmunoCAP test. Fifty-five out of seventy of the confirmed cases of CMPA showed a CoMiSS > 12 while five out of thirteen cases did not show a confirmed diagnosis of CMPA even with CoMiSS > 12 (
Table 2). This primarily suggested CoMiSS to be a particularly useful tool in diagnosing CMPA.
Table 2
Cow's Milk-related Symptom Score (CoMiSS) of infants aged 0–24 months

|
Variable |
No. (%) |
|
CoMiSS |
|
|
≤12 |
23 (27.7) |
|
>12 |
60 (72.3) |
|
Suspected CMPA is confirmed by |
|
|
ImmunoCAP method |
26 (31.3) |
|
Oral food challenge test |
47 (56.6) |
|
Not a case of cow's milk allergy |
11 (13.3) |
|
CMPA present |
|
|
Yes |
70 (84.3) |
|
|
≤12 |
15 (21.4) |
|
|
>12 |
55 (78.6) |
|
No |
13 (15.7) |
|
|
≤12 |
5 (38.5) |
|
|
>12 |
8 (61.5) |

Furthermore, CoMiSS had a positive predictive value (PPV) of 93%, negative predictive value (NPV) of 33%, with sensitivity of 77%, and specificity of 66%. A receiver operating characteristics (ROC) curve area of 0.68 at a CoMiSS cutoff of 12 was observed. The area under the curve (AUC) of the ROC curve was found to be 0.68. The thin, straight line suggests the random estimate with an AUC of 0.5 (
Fig. 2).
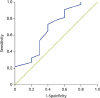 | Fig. 2The receiver operating characteristics (ROC) curve predicting the probability of cow's milk protein allergy confirmed with a skin prick test, immunoCAP test, or open oral food challenge test with a Cow's Milk-related Symptom Score arbitrary cutoff score of 12.
|
CoMiSS and its association with other factors
Immunization
Childhood immunization was one of the greatest public health successes of the last century. Vaccines contain an active component (the antigen) that induces an immune response. They may also contain additional components such as preservatives, additives, and traces of other substances. There has been an increase in mass immunization, leading to the hypothesis that certain vaccines may increase the risk of allergic disease. However, the study suggested that the mean CoMiSS was 15.58 ± 6.55 in children who were immunized, and 21.5 ± 7.6 in children who were not immunized as per their schedule (p = 0.02).
Feeding pattern
The introduction of complementary food and its relationship with allergies has been controversial. The World Health Organization suggests exclusive breastfeeding till 6 months of age, followed by the introduction of complementary foods. Early or delayed introduction of complementary foods has been attributed to increased risk of allergies such as CMPA in infants. 26 infants were exclusively breastfed for the first 6 months of life. Twenty of these infants had a CoMiSS > 12, 57 of them were not exclusive breast fed during the first 6 months of life, and 39 of them demonstrated a CoMiSS > 12 (
p = 0.3). The number of infants introduced to complementary foods before 6 months, at 6 months, and after 6 months was 55, 21, and 7; and a CoMiSS > 12 was seen in 39, 16, and 4 infants, respectively (
p = 0.42) (
Table 3). Thus, the data suggested an association between feeding pattern and CoMiSS that was not statistically significant.
Table 3
Cow's Milk-related Symptom Score (CoMiSS) and its association with infant feeding pattern

|
Variable |
CoMiSS |
p value |
|
≤12 |
>12 |
|
Exclusive breastfeeding for first 6 months |
|
|
0.3 |
|
Yes (n = 26) |
6 |
20 |
|
No (n = 57) |
18 |
39 |
|
Introduction of complementary feeding |
|
|
0.42 |
|
<6 mo (n = 55) |
16 |
39 |
|
At 6 mo (n = 21) |
5 |
16 |
|
After 6 mo (n = 7) |
3 |
4 |

Gestational age
CMPA may be associated with an infant's gestational age. The figure demonstrates the relationship between CoMiSS and gestational age. Although the major CoMiSS points lie between 36 and 40 weeks, no direct association was found between gestational age and CoMiSS (
Fig. 3).
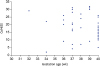 | Fig. 3Scatter plot between gestation age and Cow's Milk-related Symptom Score (CoMiSS).
|
Birth weight
CMPA may be associated with an infant's birth weight. The figure demonstrates the relationship between CoMiSS and birth weight. Although the major CoMiSS points lie between 2.5 to 3.5 kg, no direct association was found between birthweight and CoMiSS (
Fig. 4).
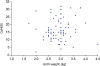 | Fig. 4Scatter plot of birth weight and Cow's Milk-related Symptom Score (CoMiSS).
|
DISCUSSION
CMPA is a rising concern in developing countries such as India [
6]. However, despite the presence of various guidelines, diagnosing CMPA is still a significant clinical challenge. The vague clinical presentation and complexity of guidelines on CMPA have a dismal effect on the identification and management of CMPA, especially among primary healthcare physicians [
457].
Gastrointestinal manifestations of CMPA are nonspecific. In infants, history and physical examination may not help distinguish GERD from CMPA. In a small group of older children, CMPA may present not only with symptoms of GERD but also with dyspepsia or abdominal pain, and hence may be easily confused with functional gastrointestinal disorders or lactose intolerance. Therefore, the challenge remains to make a correct diagnosis while minimizing the burden on the patient and family. An improper diagnostic protocol substantially increases the risk of over- and underdiagnosis, over- and undertreatment, impeding growth outcomes aong infants. Moreover, an organized diagnostic protocol favors appropriate dietary interventions, thus supporting normal growth and development. In contrast, a diet that is not indicated or continued when the child may have already developed tolerance, may impair growth and quality of life, while causing unnecessary healthcare costs. It is, therefore imperative that a systematic approach is available to avoid unnecessary suffering, improper diagnosis, and long-term health impact of CMPA [
457].
None of the diagnostic tests available in routine clinical situations prove or exclude CMPA completely. A thorough history, including family history of atopy and a careful clinical examination are therefore key elements in the diagnostic process. Clinicians may perform SPT to evaluate IgE titers. Persistently high IgE titers increase the risk of developing other atopic conditions such as asthma, rhinoconjunctivitis, and atopic dermatitis. Patch testing, still a subject of ongoing research, can aid in the diagnosis of non-IgE-mediated reactions. The intricacies associated with the assessment of these symptoms leading to improper diagnosis considerably delays a food challenge by a primary care physicians until the patient visits a pediatric specialist [
710].
Considering multiple clinical hurdles in the diagnosis of CMPA, CoMiSS was developed to help general physicians with cases of suspected CMPA and/or to evaluate the progress of symptoms associated with CMPA. Thus, this study was conducted to focus on CMPA in India, as there is a scarcity of data on CMPA in India; to increase awareness about CMPA in primary healthcaresettings; and to evaluate the application of a simple tool such as CoMiSS in the early diagnosis of CMPA [
89].
CoMiSS is a simple, fast, and easy-to-use tool to facilitate awareness and help in early diagnosis of CMPA. CoMiSS can also be used to evaluate and quantify the progression of CMPA during a therapeutic intervention. As CoMiSS takes about 5 and 15 minutes, it demonstrated its efficacy as a rapid and easy to use tool. Scores are assigned to crying, regurgitation, stool, skin and respiratory symptoms. The total scores range between 0–33. A score of 12 or above suggests a risk of CMPA, warranting further diagnostic tests [
89].
A CoMiSS > 12 was seen in 72% of the children; and overall, 84.3% of the children were diagnosed with CMPA via oral food challenge/ImmunoCAP test, which suggests that CoMiSS can be used as an easy and predictive tool in the diagnosis of CMPA. Another study conducted by Vandenplas et al. [
89] suggested that a low CoMiSS even after absence of cow's milk protein and its derivatives for one month can have a considerable risk of a positive challenge test (odds ratio, 0.83; 95% confidence interval, 0.75–0.93;
p = 0.002). Although this data is not substantial enough to deduce an inference, the findings resonate that a CoMiSS>12 may be a vital cutoff value to recognize symptoms related to CMPA in infants. In addition, its ability to hint towards a diagnosis in less time, and its ease of use by general physicians, is a vital advantage of the tool. CoMiSS can be a vital step in delaying the progression of CMPA, help in its early diagnosis, and prevent misdiagnosis; in addition, it cuts down parental anxiousness as well. Early data show the predictive value of the tool in identifying infants at risk of CMPA of 80%.
According to the 11-strong Expert Consensus Panel though primary healthcare professionals are not expected delve into the expertise of complex conditions such as CMPA, a simple tool to help address its existence with a fact-based scoring system is a key step to its early diagnosis. CoMiSS being an effortless tool is a practical method to help reduce the delays and difficulties associated with CMPA, thus reducing the ongoing stress among infants and parents as well as healthcare professionals.
The cause of CMPA is generally unknown. However, it is commonly seen children with a family history of allergies. Other causes include lack of breastfeeding, early or delayed complementary feeding and early exposure to cow's milk protein. On the contrary, evidence also suggests that breastfed infants may also develop CMPA, primarily attributed to the presence of low levels of cow's milk-specific IgA in breast milk. Other factors include maternal diet during breastfeeding, age of introduction of solid foods and allergenic foods, exposure to pollutants, cesarean section, and maternal age [
111213]. In this study, factors such as birth weight, gestational age, feeding pattern, and immunization schedule showed no correlation with CoMiSS.
Use of CoMiSS could be a way forward for overcoming clinical challenges associated with CMPA such as improving the recognition of symptoms, supporting the clinical decision-making and aiding in appropriate management of this common but overlooked condition. CoMiSS may also be useful in tracking the improvement or worsening of symptoms during a therapeutic intervention [
89].
In the Indian primary care setting, CoMiSS is suggested to have a strong predictive value and could probably be used as a comprehensive first line tool to detect CMPA in children less than two years of age, encouraging early diagnosis. The high PPV of CoMiSS cannot be disregarded thus providing the relevance of the parameters included in the tool. The low NPV could plausibly be demonstrated by the partial treatment that some patients would have undergone prior to visiting the pediatric clinics, hence confounding the score. CoMiSS may be a sensitive and precise awareness tool for healthcare professionals to select infants suspected to have cow's milk-related symptoms. However, appropriate validation studies are needed to evaluate its overall usage and its possible position in future formulated guidelines. Also, larger epidemiological studies are required to confirm its predominance.
ACKNOWLEDGEMENTS
This paper acknowledges the contribution of BioQuest Solutions in the preparation of this manuscript.
References
1. Johnson CP, Blasco PA. Infant growth and development. Pediatr Rev. 1997; 18:224–242.

3. Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. 2012; 129:1187–1197.

4. Vandenplas Y; Althera Study Group, Steenhout P, Grathwohl D. A pilot study on the application of a symptom-based score for the diagnosis of cow's milk protein allergy. SAGE Open Med. 2014; 2:2050312114523423.

5. Vandenplas Y, Koletzko S, Isolauri E, Hill D, Oranje AP, Brueton M, Staiano A, Dupont C. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Arch Dis Child. 2007; 92:902–908.

6. Allen KJ. Cow's milk protein allergy: an entity for recognition in developing countries. J Gastroenterol Hepatol. 2010; 25:4–6.

7. Lozinsky AC, Meyer R, Anagnostou K, Dziubak R, Reeve K, Godwin H, Fox AT, Shah N. Cow's milk protein allergy from diagnosis to management: a very different journey for general practitioners and parents. Children (Basel). 2015; 2:317–329.

8. Vandenplas Y, Dupont C, Eigenmann P, Host A, Kuitunen M, Ribes-Koninckx C, Shah N, Shamir R, Staiano A, Szajewska H, Von Berg A. A workshop report on the development of the Cow's Milk-related Symptom Score awareness tool for young children. Acta Paediatr. 2015; 104:334–339.

9. Vandenplas Y, Steenhout P, Järvi A, Garreau AS, Mukherjee R. Pooled analysis of the Cow's Milk-related-Symptom-Score (CoMiSS™) as a predictor for cow's milk related symptoms. Pediatr Gastroenterol Hepatol Nutr. 2017; 20:22–26.

10. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Staiano A, Schäppi MG, Vandenplas Y. European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012; 55:221–229.
11. Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995; 95:1179–1190.

12. Ferreira CT, Seidman E. Food allergy: a practical update from the gastroenterological viewpoint. J Pediatr (Rio J). 2007; 83:7–20.

13. Järvinen KM, Laine ST, Järvenpää AL, Suomalainen HK. Does low IgA in human milk predispose the infant to development of cow's milk allergy? Pediatr Res. 2000; 48:457–462.








 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download