This article has been
cited by other articles in ScienceCentral.
Abstract
Food allergy has an estimated prevalence of 6%–8% in children. Meat allergy and multiple food allergy due to sensitization to cross-reactive components in infancy is, however, less frequent. A 5-year-old girl was referred to our department with a multiple food allergy history. She had severe immediate worsening of her atopic dermatitis with hen's egg (6 months) and cow's milk introduction (7 months). At the age of 9 months, she presented with recurrent and reproducible atopic dermatitis' worsening and lip edema with the introduction of different meats (chicken, turkey, cow, pork, and rabbit), having the same complaints with fish at 12 months (salmon and hake). At her first appointment she was avoiding hen's egg, cow's milk, meat, and fish (except fresh tuna, codfish, and pollock). We performed skin prick tests (commercial extract and prick-to-prick with whole food) and specific IgE, which revealed sensitization to hen's egg, raw meat (cow, pork, chicken, turkey, duck, lamb, goat, and rabbit; negative for cooked meat), codfish and cow's milk (mild). ISAC was performed, revealing sensitization to 3 cross-reactive components (serum albumins Bosd6, Canf3, and Feld2) and specific food components of chicken's egg/meat (Gald1, 2, 3, and 5), cod (Gadc1), hazelnut (Cora9), and kiwi (Actd1). We present a rare case of multiple food allergy in infancy, where sensitization to cross-reactive components was responsible for most of the children complaints. The detection of serum albumins' involvement was especially important, because it can possibly mean tolerance to these foods in well-cooked forms, substantially improving patient and family's quality of life.
Keywords: Food allergy, Serum albumin, Cross-reactions
INTRODUCTION
Food allergy has an overall estimated prevalence of 6%–8% in children, being cow's milk, hen's egg, fish, wheat, soy, peanuts, and tree nuts the most frequent allergens involved [
1]. Meat allergy and multiple food allergy due to sensitization to cross-reactive components in infancy is, however, less frequent.
Serum albumins (SAs) are α-helical globular proteins synthesized in the liver, being abundant in plasma and contributing to regulation of oncotic pressure. They're present in dander, saliva, milk, and meat of animals, being denatured by heating due to their thermolabile properties [
23]. SAs are usually considered minor allergens, with seven being officially registered as allergens by World Health Organization and International Union of Immunological Societies Allergen Nomenclature Subcommittee: Bos d 6 (bovine), Can f 3 (dog), Cav p 4 (guinea pig), Equ c 3 (horse), Fel d 2 (cat), Gal d 5 (chicken), and Sus s 1 (pig). Mammalian SAs have high sequence identities and similarities (72%–88%) relative to human SA (HSA) and between them, while aviarian SA presents lower identities (42%–49%) to HSA and other mammalian SA [
2]. Due to their high sequence identity (>70%) mammalian SAs are highly cross-reactive [
2], while cross-reactivity between mammalian and aviarian SA is rare but already described [
4].
CASE REPORT
We report the case of a 5-year-old girl that was referred by her pediatrician to our Imunoallergology Department for investigation of a multiple food allergy. She had previous clinical history of moderate atopic dermatitis (AD) since 3 months, moderate to severe persistent allergic rhinitis since 3 years and recurrent wheezing since the age of 4, with sensitization to house dust mites, mold, grass pollen, cat epithelium and feathers. She was medicated with daily emollient, nasal corticosteroid, oral antihistamine, leukotriene antagonist, and inhaled long-acting β-2 agonist/corticosteroid association. Her father had AD and her sister of 12-years-old has AD, allergic rhinitis and asthma. She lives in an urban environment without domestic pets but with frequent contact with pigeons outside and neighbor's cat. She had severe immediate worsening of her AD with hen's egg (white and yolk simultaneously) and cow's milk introduction (without previous complaints with formula milk) at 6 and 7 months, respectively. At the age of 9 months she presented with recurrent and reproducible AD's worsening and lip edema with the introduction of different meats (chicken, turkey, cow, pork, and rabbit). The same happened at 12 months with fish (salmon and hake). Her mother denies AD's worsening or other complaints outside these episodes related with food intake. She was referred several times to our department before but she missed all the appointments. At her first appointment she was 5-years-old and she was avoiding hen's egg, cow's milk (tolerated milk traces), meat, and fish (except fresh tuna, codfish, and pollock).
We performed skin prick tests (SPT) (commercial extract and prick-to-prick with whole food) with all the suspected foods (
Fig. 1). They were considered positive when showing a wheal with the largest diameter at least 3 mm larger than that of the negative control. SPT were positive (
Fig. 2) for hen's egg (white and yolk – commercial extract and food in nature, raw and cooked forms; ovomucoid; ovalbumin) and raw meat (cow, pork, chicken, turkey, duck, lamb, goat, and rabbit; negative for cooked meat). SPT were negative for all of the other foods suspected.
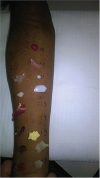 | Fig. 1 Picture of skin prick tests (commercial extract and prick-to-prick with whole food) with cow's milk, hen's egg, fish, and meat (raw and cooked).
|
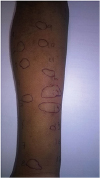 | Fig. 2 Picture of skin prick tests results - positive results for hen's egg (white and yolk) and raw meat (cow, pork, turkey, and rabbit); negative results for cooked meat, cow's milk, and codfish.
|
She had total IgE of 1,078 kU/L. Specific IgE (ImmunoCAP system, Thermo Fisher, Uppsala, Sweden) was strongly positive for hen's egg (all components) and chicken meat, slightly positive for pork meat, codfish and cow's milk and fractions and negative for alpha-gal (
Fig. 3A). ISAC (Thermo Fisher) was performed (
Fig. 3B), revealing sensitization to 3 cross-reactive components (SAs Bos d 6, Can f 3, and Fel d 2) and specific food components of chicken's egg/meat (Gal d 1-3 and 5), codfish (Gad c 1), hazelnut (Cor a 9), and kiwi (Act d 1), the last two without associated clinical manifestations.
 | Fig. 3 In vitro workup. (A) Specific IgE (ImmunoCAP system, Thermo Fisher, Uppsala, Sweden) results - only positive results were presented. (B) ISAC (Thermo Fisher) results. B. tropicalis, Blomia tropicalis; D. farinae, Dermatophagoides farinae; D. pteronyssinus, Dermatophagoides pteronyssinus; L. destructor, Lepidoglyphus destructor.
|
Until present time she still maintains sensitization and is still avoiding hen's egg but she can now tolerate lamb, goat, and duck meat and cow's milk in very well cooked form. Fish allergy is now overcome.
DISCUSSION
In this case sensitization to bovine SA (BSA) Bos d 6 seems to be responsible for cow's milk and beef allergy. In fact BSA has been identified as one of major beef allergens, particularly in children, being responsible for clinical cross-reactivity between raw beef and cow's milk (sensitization independent of other cow's milk proteins, as seen in this case) [
567]. Sensitization to other SAs may be explained by the high sequence homology between mammalian SAs, being possibly responsible for the allergic symptoms reported with pork and rabbit meat (SAs not available). It is important, however, to note that patient presented sensitization to cat epithelium and this may also have contributed to pork meat sensitization, by cross-reactivity between cat and pork SAs (cat-pork syndrome), as already been reported in literature [
89]. Regarding complaints with chicken and turkey meat, since cross-reactivity between aviarian and mammalian SA is rare [
2], these are probably due to sensitization to Gal d 5. Primary sensitization to Gal d 5, a food and inhalant allergen, may have occurred in this case through 2 different routes: respiratory, with evidence of sensitization to feathers (bird-egg syndrome) or alimentary due to hen's egg allergy, with evidence of sensitization to Gal d 1-3 (egg-bird syndrome) [
10].
This case report has some limitations, such as some degree of subjectivity in clinical symptoms (mainly AD' worsening) and the inability to perform the oral food challenge until present time due to different reasons.
In conclusion we present a rare case of multiple food allergy in infancy, where sensitization to cross-reactive components was responsible for most of the children complaints. The discovery of SAs' involvement is especially important in similar cases as the one we presented because it may enable a possible tolerance to well-cooked meat and boiled milk, as described in this case, which will lead to a substantially improvement of the patient and family's quality of life.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download