Abstract
Background
The global prevalence of allergic rhinitis, asthma, and atopic dermatitis has risen significantly over the last 2 decades. Allergic sensitization to aeroallergen is a major risk factor in developing the allergic disease. The prevalence of aeroallergen sensitization varies in different regions and countries.
Objective
To determine the prevalence of common aeroallergen sensitization and the atopic status among adult patients.
Methods
A cross-sectional, retrospective study. The data were collected from medical records and database of the result of skin prick test of patients who had the allergic symptoms or chronic urticaria in adult allergy clinic, Ramathibodi hospital from January 2004 to December 2015.
Results
A total of 1,516 of patients (female, 1,118 [73.7%]) were enrolled. The mean ages of participants were 41.34 (standard deviation, ±16.5) years. Fifty-eight percent (58%) of patients were diagnosed with allergic rhinitis, 19.7%, 3.2%, and 9.2% with asthma, atopic dermatitis, and chronic urticaria respectively. In the chronic urticaria group, 57.4% underwent the positive skin prick test to common aeroallergens. Mites were responsible for the most common inhaled allergen sensitization in this study as 50.1% of Dermatophagoides pteronyssinus, 32% of Dermatophagoides farinae, and 31.5% of house dust. Cockroach was the second most common aeroallergen sensitization as 32.3% followed by grass pollen, Bermuda (21.1%) and timothy (13.6%). The animal dander, cat and dog, occupied 12.9 and 10% respectively.
Go to : 
References
1. Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK. Williams HIsaac Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006; 368:733–43.

2. Pearce N, Ait-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E. Robertson CIsaac Phase Three Study Group. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007; 62:758–66.

3. Bjorksten B, Clayton T, Ellwood P, Stewart A. Strachan DIsaac Phase III Study Group. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008; 19:110–24.

4. Bunnag C, Jareoncharsri P, Tantilipikorn P, Vichyanond P, Pawankar R. Epidemiology and current status of allergic rhinitis and asthma in Thailand – ARIA Asia-Pacific Workshop report. Asian Pac J Allergy Immunol. 2009; 27:79–86.
5. Wong GW, Leung TF, Ko FW. Changing prevalence of allergic diseases in the Asia-pacific region. Allergy Asthma Immunol Res. 2013; 5:251–7.

6. Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017; 35:283–9.

7. Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol. 2007; 120:381–7.

8. Cookson WO, Sharp PA, Faux JA, Hopkin JM. Linkage between immunoglobulin E responses underlying asthma and rhinitis and chromosome 11q. Lancet. 1989; 1:1292–5.

9. Allen M, Heinzmann A, Noguchi E, Abecasis G, Broxholme J, Ponting CP, Bhattacharyya S, Tinsley J, Zhang Y, Holt R, Jones EY, Lench N, Carey A, Jones H, Dickens NJ, Dimon C, Nicholls R, Baker C, Xue L, Townsend E, Kabesch M, Weiland SK, Carr D, von Mutius E, Adcock IM, Barnes PJ, Lathrop GM, Edwards M, Moffatt MF, Cookson WO. Positional cloning of a novel gene influencing asthma from chromosome 2q14. Nat Genet. 2003; 35:258–63.

10. Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008; 8:169–82.

11. Le Souef PN. Variations in genetic influences on the development of asthma throughout childhood, adolescence and early adult life. Curr Opin Allergy Clin Immunol. 2006; 6:317–22.

12. Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, Heederik D, Piarroux R. von Mutius EGabriela Transregio 22 Study Group. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011; 364:701–9.

13. Illi S, Depner M, Genuneit J, Horak E, Loss G, Strunz-Lehner C, Buchele G, Boznanski A, Danielewicz H, Cullinan P, Heederik D, Braun-Fahrlander C. von Mutius EGabriela Study Group. Protection from childhood asthma and allergy in Alpine farm environments-the GABRIEL Advanced Studies. J Allergy Clin Immunol. 2012; 129:1470–7. e6.

14. Marfortt DA, Josviack D, Lozano A, Cuestas E, Aguero L, Castro-Rodriguez JA. Differences between preschoolers with asthma and allergies in urban and rural environments. J Asthma. 2018; 55:470–6.

15. Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001; 108:E33.

16. Kihlstrom A, Lilja G, Pershagen G, Hedlin G. Exposure to birch pollen in infancy and development of atopic disease in childhood. J Allergy Clin Immunol. 2002; 110:78–84.

17. Ghunaim N, Wickman M, Almqvist C, Soderstrom L, Ahlstedt S, van Hage M. Sensitization to different pollens and allergic disease in 4-year-old Swedish children. Clin Exp Allergy. 2006; 36:722–7.

18. Illi S, von Mutius E, Lau S, Nickel R, Niggemann B, Sommerfeld C. Wahn UMulticenter Allergy Study Group. The pattern of atopic sensitization is associated with the development of asthma in childhood. J Allergy Clin Immunol. 2001; 108:709–14.

19. Ronmark E, Bjerg A, Perzanowski M, Platts-Mills T, Lundback B. Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden. J Allergy Clin Immunol. 2009; 124:357–63. 63.e1-15.

20. Sakashita M, Hirota T, Harada M, Nakamichi R, Tsunoda T, Osawa Y, Kojima A, Okamoto M, Suzuki D, Kubo S, Imoto Y, Nakamura Y, Tamari M, Fujieda S. Prevalence of allergic rhinitis and sensitization to common aeroallergens in a Japanese population. Int Arch Allergy Immunol. 2010; 151:255–61.

21. Miraglia Del Giudice M, Pedulla M, Piacentini GL, Capristo C, Brunese FP, Decimo F, Maiello N, Capristo AF. Atopy and house dust mite sensitization as risk factors for asthma in children. Allergy. 2002; 57:169–72.

22. Pefura-Yone EW, Kengne AP, Kuaban C. Sensitisation to mites in a group of patients with asthma in Yaounde, Cameroon: a cross-sectional study. BMJ Open. 2014; 4:e004062.

23. Resch Y, Michel S, Kabesch M, Lupinek C, Valenta R, Vrtala S. Different IgE recognition of mite allergen components in asthmatic and nonasthmatic children. J Allergy Clin Immunol. 2015; 136:1083–91.

24. Vidal C, Lojo S, Juangorena M, Gonzalez-Quintela A. Association between asthma and sensitization to allergens of Dermatophagoides pteronyssinus. J Investig Allergol Clin Immunol. 2016; 26:304–9.

25. Trakultivakorn M, Sangsupawanich P, Vichyanond P. Time trends of the prevalence of asthma, rhinitis and eczema in Thai children-ISAAC (International Study of Asthma and Allergies in Childhood) Phase Three. J Asthma. 2007; 44:609–11.

26. Liutu M, Kalimo K, Uksila J, Kalimo H. Etiologic aspects of chronic urticaria. Int J Dermatol. 1998; 37:515–9.

27. Caliskaner Z, Ozturk S, Turan M, Karaayvaz M. Skin test positivity to aeroallergens in the patients with chronic urticaria without allergic respiratory disease. J Investig Allergol Clin Immunol. 2004; 14:50–4.
28. Mahesh PA, Kushalappa PA, Holla AD, Vedanthan PK. House dust mite sensitivity is a factor in chronic urticaria. Indian J Dermatol Venereol Leprol. 2005; 71:99–101.

29. Kulthanan K, Jiamton S, Rutnin NO, Insawang M, Pinkaew S. Prevalence and relevance of the positivity of skin prick testing in patients with chronic urticaria. J Dermatol. 2008; 35:330–5.

30. Toukhsati SR, Phillips CJC, Podberscek AL, Coleman GJ. Companion animals in Thailand: human factors that predict sterilization of cats and dogs. Soc Anim. 2015; 10:569–93.
Go to : 
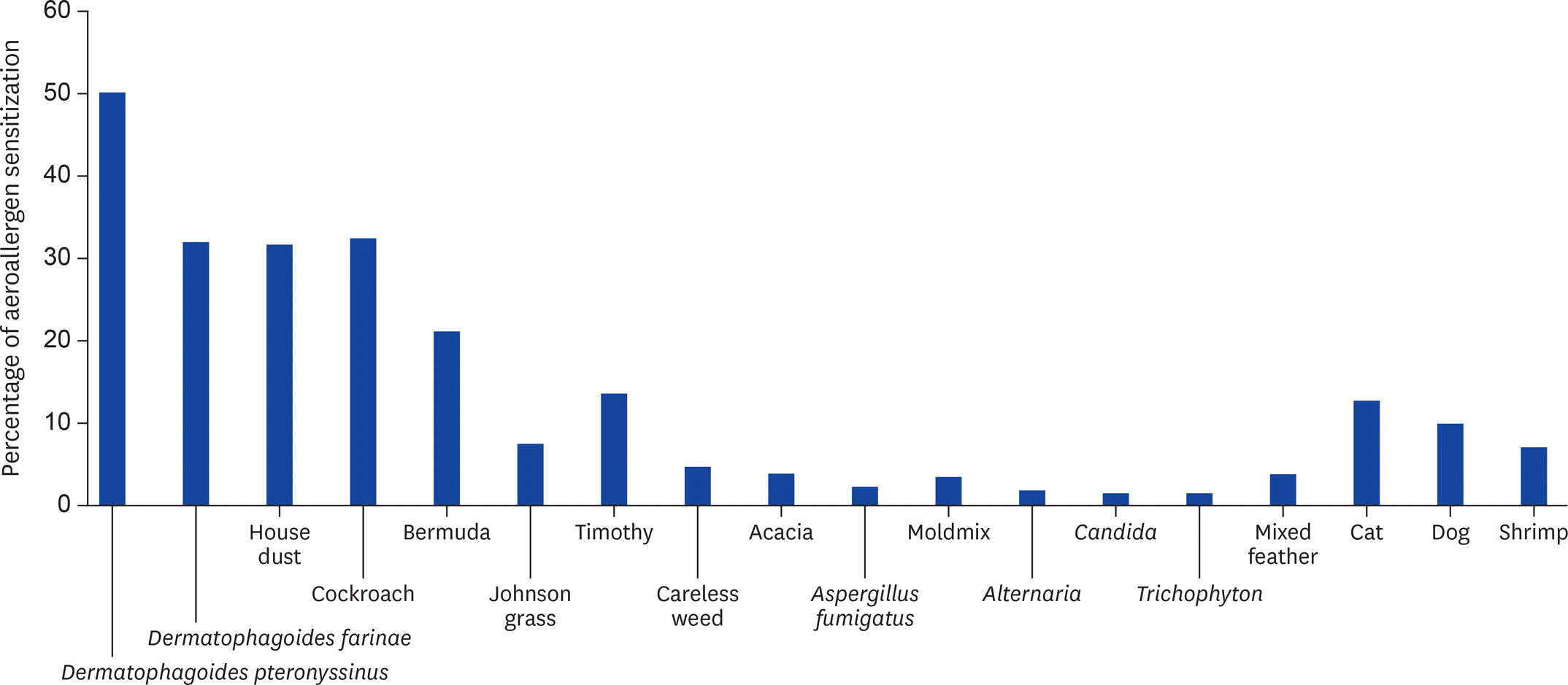 | Fig. 1.Prevalence of common aeroallergen sensitization in a tertiary care hospital, Thailand. |
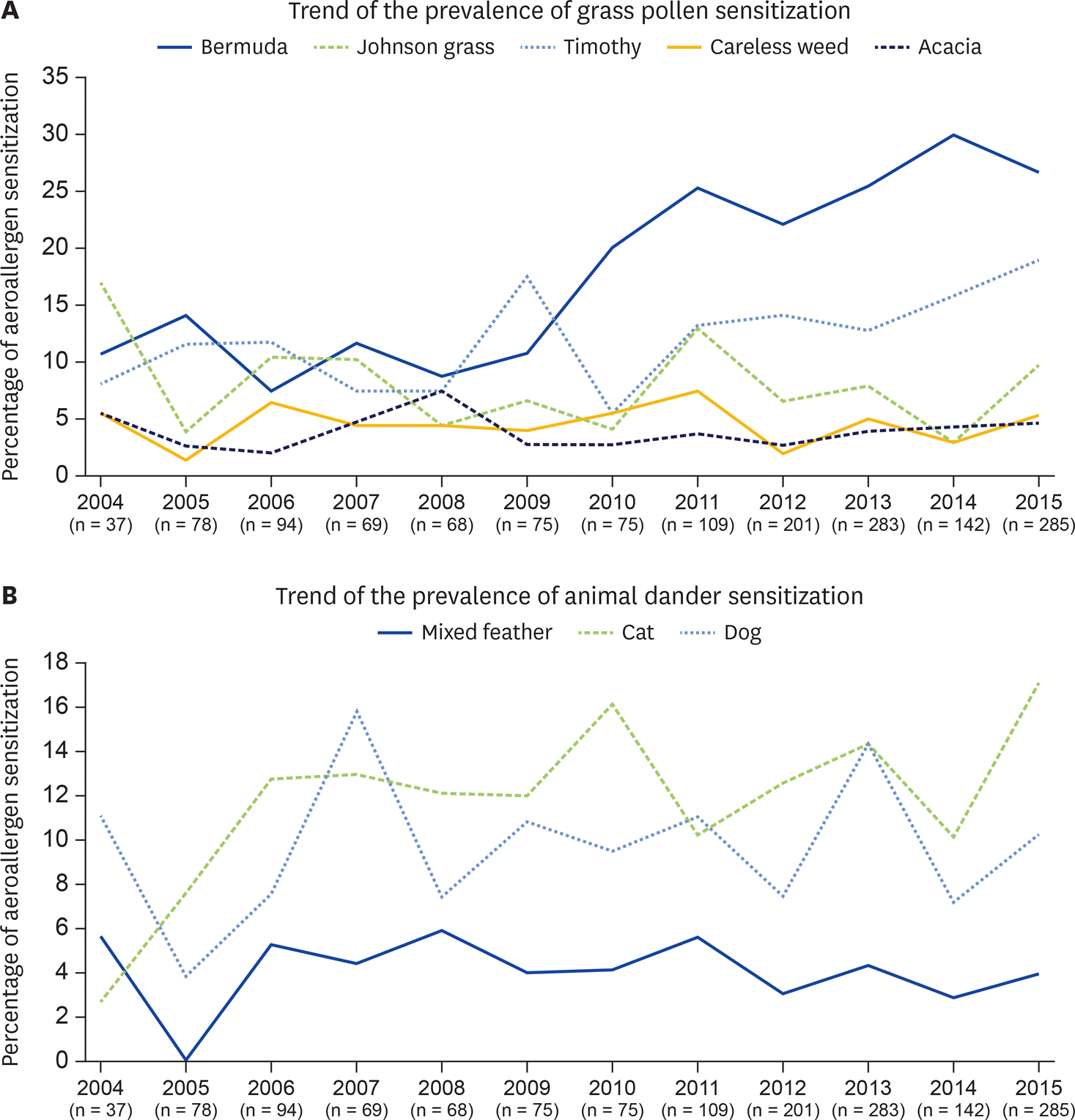 | Fig. 2.Changing trend of the prevalence of common aeroallergen sensitization over a 12-year period in a tertiary care hospital, Thailand. (A) Trend of the prevalence of grass pollen sensitization. (B) Trend of the prevalence of animal dander sensitization. (C) Trend of the prevalence of mites, house dust and cockroach sensitization. (D) Trend of the prevalence of fungal sensitization. (continued to the next page) |
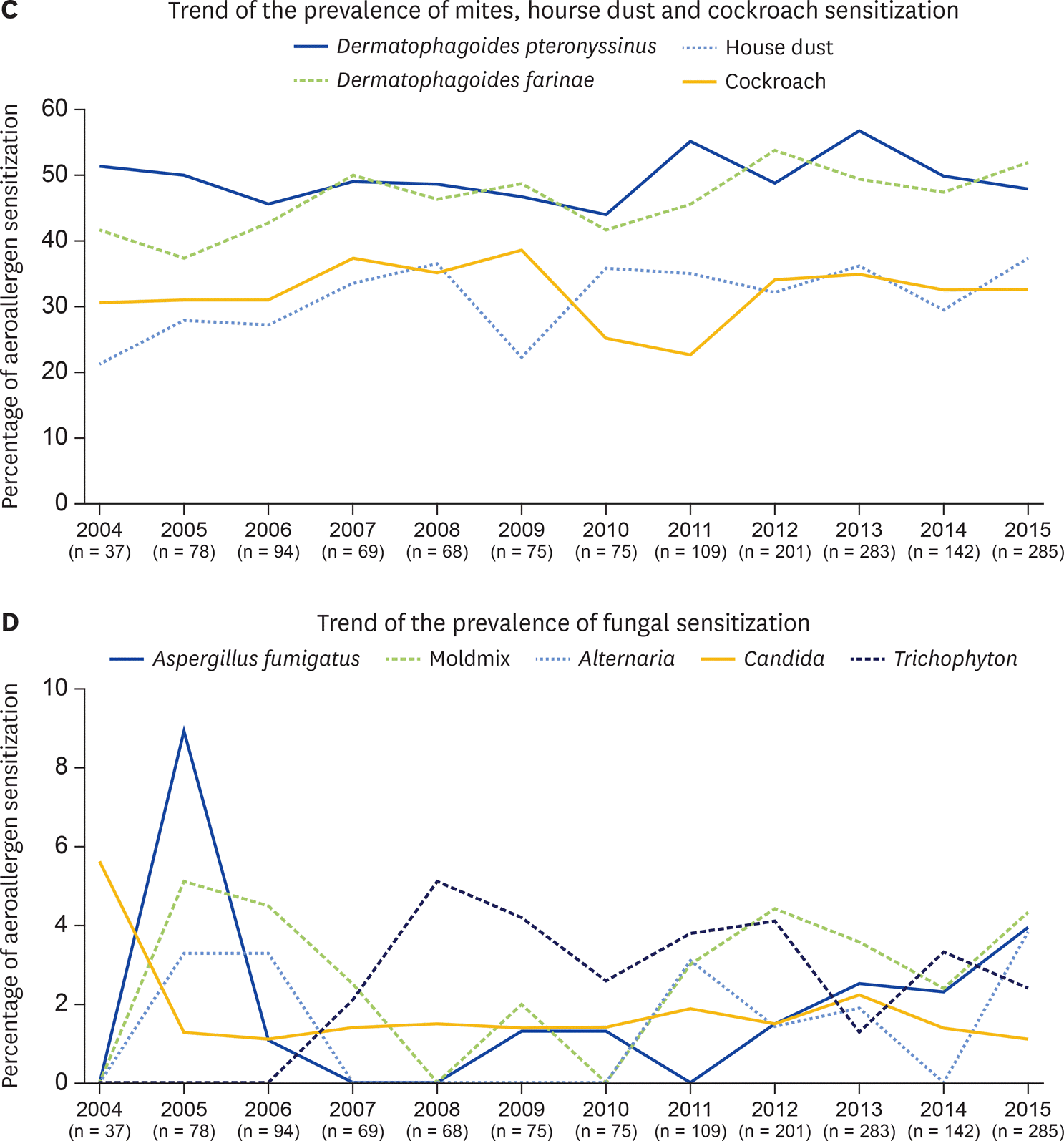 | Fig. 2.Changing trend of the prevalence of common aeroallergen sensitization over a 12-year period in a tertiary care hospital, Thailand. (A) Trend of the prevalence of grass pollen sensitization. (B) Trend of the prevalence of animal dander sensitization. (C) Trend of the prevalence of mites, house dust and cockroach sensitization. (D) Trend of the prevalence of fungal sensitization. (continued to the next page) |
Table 1.
Demographic data of 1,516 patients in adult allergy clinic from 2004 to 2015 in a tertiary care hospital, Thailand




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download