INTRODUCTION
MATERIALS AND METHODS
Patient selection
Materials
Clinical parameters
0=no dental plaque;
1=the presence of a discontinuous line of dental plaque at the gingival margin;
2=a continuous line of dental plaque at the gingival margin that does not extend further than 1 mm from the margin;
3=dental plaque coverage that is greater than 1 mm, but does not extend further than one-third of the tooth;
4=dental plaque that covers more than one-third but not more than two-thirds of the tooth surface;
5=dental plaque coverage over more than two-thirds of the tooth surface.
0=normal gingiva;
1=mild inflammation: slight change in the color and slight edema; no bleeding on probing;
2=moderate inflammation: redness, edema, and glazing; bleeding on probing;
3=severe inflammation: marked redness and edema; ulceration; tendency for spontaneous bleeding.
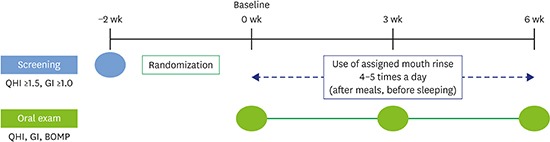
Experimental design
 | Figure 1Overview of the experimental protocol.
QHI: Turesky-Quigley-Hein plaque index, GI: Löe-Silness gingival index, BOMP: bleeding on marginal probing.
|
Table 1
Overview of the experimental protocol

| Parameters | Visit 0 (−2 wk) | Visit 1 (baseline) | Visit 2 (3 wk) | Visit 3 (6 wk) |
|---|---|---|---|---|
| Informed consent | ○ | - | - | - |
| Subject selection | ○ | - | - | - |
| Oral hygiene instruction | ○ | - | - | - |
| QHI | ○ | ○ | ○ | ○ |
| GI | ○ | ○ | ○ | ○ |
| BOMP | - | ○ | ○ | ○ |
Statistical analysis
RESULTS
Table 2
Background information of the patients
QHI
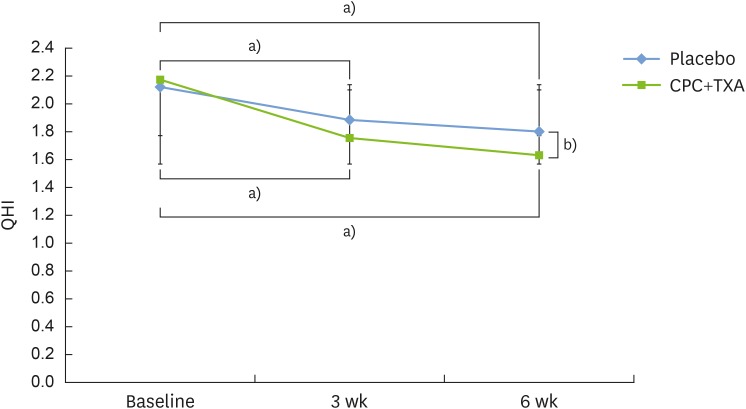 | Figure 2The QHI. Statistically significant differences were evident after 3 weeks of treatment for both groups (experimental group: CPC and TXA, and placebo control group). At 6 weeks, a significant difference was observed in the intergroup analysis. There was a statistically significant difference in the values after 6 weeks of treatment for both groups. Bars represent mean±standard deviation.
QHI: Turesky-Quigley-Hein plaque index, CPC: cetylpyridinium chloride, TXA: tranexamic acid.
a)Statistically significant intragroup difference compared to the baseline, according to the Friedman test; b)Statistically significant intergroup difference between the 2 groups at 6 weeks, according to the Mann-Whitney U test.
|
Table 3
Mean values of the clinical parameters
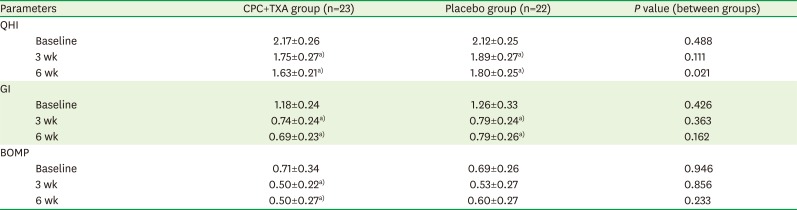
GI
 | Figure 3The GI. A statistically significant difference was evident after 3 weeks of using the CPC and TXA mouthwash. Statistically significant differences were found between the values at baseline and those at 6 weeks for both groups. Bars represent mean±standard deviation.
GI: Löe-Silness gingival index, CPC: cetylpyridinium chloride, TXA: tranexamic acid.
a)Statistically significant intragroup difference compared to baseline, according to the Friedman test.
|
BOMP
 | Figure 4The incidence of gingival BOMP. In the CPC and TXA group, statistically significant differences were observed at 3 weeks and at 6 weeks.
CPC: cetylpyridinium chloride, TXA: tranexamic acid, BOMP: bleeding on marginal probing.
a)Statistically significant intragroup difference compared to baseline, according to the Friedman test.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download