Abbreviations
CMI
FBS
gE
HZ
IE
IEDB
IFN
MHC
mIL-33
mIL-7
OLP
PFU
TNF
VV-gE
VZV
INTRODUCTION
MATERIALS AND METHODS
Animals
DNA plasmids
Immunization
Overlapping peptides
Interferon (IFN)-γ ELISPOT assays
Intracellular cytokine staining
Epitope mapping and major histocompatibility complex (MHC) class Ι dextramer staining
Recombinant vaccinia virus challenge and plaque assays
Statistical analysis
RESULTS
HZ DNA vaccines induce VZV protein-specific T-cell immune responses
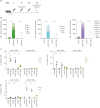 | Figure 1T-cell immunogenicity of HZ DNA vaccines in C57BL/6 mice. Five to six-week-old female C57BL/6 mice were intramuscularly immunized three times at 2-week intervals with 30 µg pVAX1-gE, pVAX1-IE63, or pVAX1-IE62 in 100 µl of PBS, followed by in vivo electroporation (n=5 for each group). The control mice were immunized with 30 µg pVAX1 (n=3). The mice were sacrificed 2 weeks after the last immunization. (A) Immunization schedule. (B) IFN-γ ELISpot assays were performed to detect antigen-specific, IFN-γ-producing T cells by stimulating splenocytes from immunized mice with OLP pools corresponding to the immunized VZV ORF or VZV-infected cell lysate. (C) Intracellular cytokine staining for IFN-γ, TNF, and IL-2 was performed to detect antigen-specific, cytokine-producing CD4+ and CD8+ T cells by stimulating splenocytes from immunized mice with the immunodominant OLP pool for each VZV antigen protein (gE-11-41, IE63-228-54, and IE62-375-111). Data are presented as means and standard deviations.
*p<0.05.
|
IL-7 and IL-33 adjuvants enhance HZ DNA vaccine-induced T-cell immunity
 | Figure 2Adjuvant effects of IL-7 and IL-33 on HZ DNA vaccine-induced CD4+ T-cell immunity. Five to 6-week-old female C57BL/6 mice were intramuscularly immunized three times at 2-week intervals with 30 µg pVAX1-gE combined with 30 µg pVAX1-mIL-7 or pVAX1-mIL-33 in 100 µl of PBS, followed by in vivo electroporation (n=5 for each group). The control mice were immunized with 30 µg pVAX1 (n=3). Intracellular cytokine staining for IFN-γ, TNF, and IL-2 was performed to detect gE-specific, cytokine-producing CD4+ T cells by stimulating splenocytes from immunized mice with the gE-11-41 OLP pool. (A) Representative FACS dot plots. (B) Percentages of each cytokine-producing cell among CD4+ T cells for IFN-γ, TNF, and IL-2. Data are presented as means and standard deviations. (C) Pie graphs show the fraction of CD4+ T cells positive for a given number of functions. (D) CD4+ T cell polyfunctionality analyzed by every possible combination of functions and compared among groups. Means are presented.
*p<0.05; ***p<0.001.
|
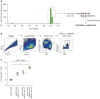 | Figure 3Adjuvant effects of IL-7 and IL-33 on HZ DNA vaccine-induced CD8+ T-cell immunity. (A, B) Five to six-week-old female C57BL/6 mice were intramuscularly immunized three times at 2-week intervals with 30 µg pVAX1-IE62 in 100 µl of PBS, followed by in vivo electroporation. IFN-γ ELISpot assays were performed by stimulating splenocytes from immunized mice with a single OLP (OLP #75–#111) from the immunodominant IE62-375-111 pool, and OLPs with high numbers of IFN-γ spots were identified. The epitope sequence, RALINLIYC (IE62527-535), with high-affinity binding to mouse MHC class Ι, H-2Db was identified by IEDB analysis, and the corresponding H-2Db IE62527-535 dextramer synthesized (A). IE62527-535-specific CD8+ T cells were detected by staining splenocytes from the pVAX1-IE62-immunized mice using H-2Db IE62527-535 dextramer (B). (C) Five to six-week-old female C57BL/6 mice were intramuscularly immunized three times at 2-week intervals with 30 µg pVAX1-IE62 combined with 30 µg pVAX1-mIL-7 or pVAX1-mIL-33 in 100 µl of PBS, followed by in vivo electroporation (n=3 for each group). Percentages of IE62527-535-specific cells among spleen CD8+ T cells were analyzed by staining splenocytes using H-2Db IE62527-535 dextramer and compared among groups. Data are presented as means and standard deviations.
*p<0.05.
|
T-cell responses elicited by HZ DNA vaccination are protective against a surrogate infection
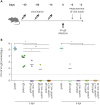 | Figure 4HZ DNA vaccine-induced protective immunity against a surrogate infection. Five to 6-week-old female C57BL/6 mice were intramuscularly immunized three times at 2-week intervals with 30 µg pVAX1-gE combined with 30 µg pVAX1-mIL-7 or pVAX1-mIL-33 in 100 µl of PBS, followed by in vivo electroporation (n=5 for each group). The control mice were immunized with 30 µg pVAX1 (n=3). The immunized mice were intraperitoneally challenged with 1x107 PFU VV-gE 2 weeks after the last immunization. (A) The schedule of immunization and viral challenge is presented. (B) Viral loads (PFU) in ovaries were measured 2 and 5 days post-infection. Means are presented.
*p<0.05; **p<0.01.
|
Single dose of HZ DNA vaccines boosts T-cell immunity in mice previously exposed to VV-gE
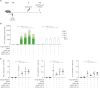 | Figure 5Immune-boosting effects of HZ DNA vaccines in mice previously infected with VV-gE. Five to 6-week-old female C57BL/6 mice were intraperitoneally infected with 1x107 PFU VV-gE. Sixty days later, the mice were intramuscularly immunized with 30 µg pVAX1-gE combined with 30 µg pVAX1-mIL-7 or pVAX1-mIL-33 in 100 µl of PBS, followed by in vivo electroporation (n=5 for each group). The control mice were immunized with 30 µg pVAX1 (n=5). The mice were sacrificed 2 weeks after the immunization. (A) Schedule of infection and immunization. (B) IFN-γ ELISpot assays were performed to detect antigen-specific, IFN-γ-producing T cells by stimulating splenocytes from the immunized mice with gE OLP pools (gE-11-41, gE-242-82, and gE-383-123) or VZV-infected cell lysate. (C) Intracellular cytokine staining for IFN-γ, TNF, and IL-2 was performed to detect antigen-specific, cytokine-producing CD4+ and CD8+ T cells by stimulating splenocytes from immunized mice with the gE-11-41 OLP pool. Data are presented as means and standard deviations.
**p<0.01.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download