INTRODUCTION
MATERIALS AND METHODS
Animal experiments
Preparation of acid-hydrolyzed silk peptide
Experimental materials
Abs and flow cytometric analyses
Lymphocyte proliferation
Analysis of cytokine expression
Determination of NK cell activity
Statistical analyses
RESULTS
Stimulation of splenocytes with silk peptide enhances NK cell activity
 | Figure 1Effect of ex vivo silk peptide treatment of splenocytes on NK cell cytolytic activity against YAC-1 target cells. Splenocytes were incubated with the indicated concentrations of silk peptide for (A) 48 h or (B) 72 h and incubated with PKH-26-labeled YAC-1 target cells at a ratio of 10:1. The degree of target cell lysis was measured as described in the Materials and Methods. Data are presented as the mean±SE (n=3) of three independent experiments.E:T, effector to target ratio.
***p<0.001, ****p<0.0001 indicate a significant difference compared to the control.
|
Silk peptide enhances the proliferation of pre-stimulated splenocytes
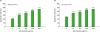
 | Figure 2Influence of oral administration of silk peptide on mitogen- or IL-2-mediated proliferation of splenocytes. Mice were orally administered various amounts of silk peptide-supplemented feed for 2 months and splenocytes were prepared. The level of (A) Con A-, (B) LPS-, or (C) IL-2-mediated proliferation of splenocytes was measured as described in the Materials and Methods. Data are presented as the mean±SE (n=3) of three independent experiments.
*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001 indicate a significant difference compared to the control (without silk peptide treatment).
|
Oral administration silk peptide in mice increases NK cell frequency and maturation
 | Figure 3Influence of oral administration of silk peptide on NK cell frequency and the status of NK cell maturation in splenocytes. Changes in (A) NK cell frequency (CD3−NK1.1+) and (B) mature NK cells (CD11bhighCD27low) in splenocytes prepared from mice orally administered various amount of silk peptide were analyzed as described in the Materials and Methods. FACS plot shows a representative result and data shown in bar graph are presented as the mean±SE (n=3) of three independent experiments.FSC-A, forward-scatter area; SSC-A, side-scatter area.
*p<0.05 and **p<0.01 indicate a significant difference compared to the control (without silk peptide treatment).
|
Oral administration of silk peptide enhances NK cell-mediated target cell lysis
 | Figure 4Cytolytic activity of NK cells purified from splenocytes. NK cells were purified from splenocytes prepared from mice fed various amounts of silk peptide and stimulated with YAC-1 target cells at a ratio of 5:1 in the presence of brefeldin A and monensin. NK cell cytolytic activity was determined by measuring (A) cell surface CD107a expression and (B) intracellular IFN-γ expression as described in the Materials and Methods. FACS plot shows a representative result and data shown in bar graph are presented as the mean±SE (n=3) of three independent experiments. NC and PC show the minimum and maximum levels, respectively.FSC-A, forward-scatter area; SSC-A, side-scatter area; NC, negative control (unstimulated control containing NK cell only without target cells); PC, positive control (NK cells stimulated with PMA and ionomycin).
*p<0.05 and **p<0.01 indicate a significant difference compared to the control (without silk peptide treatment).
|
Silk peptide treatment stimulates the expression of cytokines that impact Th1-type immune responses
 | Figure 5Level of cytokine expression following stimulation of splenocytes prepared from mice fed various amount of silk peptide with PMA and ionomycin. Splenocytes prepared from silk peptide-treated mice were stimulated in vitro with PMA and ionomycin. The expression levels of the Th1-type cytokines (A) IFN-γ and (B) IL-2, and the Th2-type cytokine (C) IL-4 were determined as described in the Materials and Methods. Data are presented as the mean±SE (n=3) of three independent experiments.
*p<0.05 indicates a significant difference compared to the control (without silk peptide treatment).
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download