INTRODUCTION
MATERIALS AND METHODS
Experimental animals and genotyping
Protocol for DSS-induced colitis
Histological assessment of colitis
Myeloperoxidase (MPO) activity assay
Western blotting
Quantitative real-time PCR (RT-PCR)
Flow cytometric analysis
Serum cytokine immunoassay
Statistical analysis
RESULTS
Clinical activity was decreased in the DSS-induced colitis STAT3 KO mouse model
 | Figure 1Clinical activity was attenuated in a DSS-induced colitis STAT3 KO mouse model. (A) Experimental design for DSS-induced colitis. (B) Graph showing body weight changes in control and DSS-treated WT and KO mice (n=7 mice, group×time interaction: p=0.2394, group effect: p<0.001, time effect: p<0.001; 2-way ANOVA with Bonferroni correction). (C) Graph showing the DAI of control and DSS-treated WT and KO mice (n=7 mice, group×time interaction: p<0.001, group effect: p<0.001, time effect: p<0.001; 2-way ANOVA with Bonferroni correction). (D) Graph showing the survival rates of control and DSS-treated WT and KO mice (n=7 mice). Data are presented as the means±SEM.
***p<0.001.
|
T cell-specific deletion of STAT3 reduced the histological score and MPO activity in mice
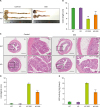 | Figure 2Deletion of STAT3 in T cells reduced histological scores and MPO activity in mice. (A) Representative images of colon tissues from control and DSS-treated WT and KO mice. (B) Bar graph for the colon lengths in control and DSS-treated WT and KO mice (n=7 mice). (C) Representative images of colon slices from control and DSS-treated WT and KO mice (scale bars: 4× image=500 μm; 20× image=100 μm). (D) Bar graph for the histological scores of control and DSS-treated WT and KO mice (n=7 mice). (E) Bar graph for the MPO activity in control and DSS-treated WT and KO mice (n=7 mice). Data are presented as the means±SEM.
*p<0.05, ***p<0.001 compared with the DSS-treated group; ###p<0.001.
|
Changes in the T lymphocyte population in T cell-specific STAT3 KO mice
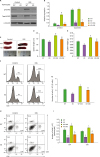 | Figure 3Changes in the T lymphocyte population in spleen of T cell-specific STAT3 KO mice. (A) Western blot analysis of pY-STAT3, total-STAT3, and α-tubulin in thymocytes from control and DSS-treated WT and KO mice. (B) Graph for the quantification of Western blot data (n=3 mice). (C) Representative images of spleen tissues from control and DSS-treated WT and KO mice. (D) Bar graph for the spleen lengths in control and DSS-treated WT and KO mice (n=7 mice). (E) Bar graph for the spleen weights in control and DSS-treated WT and KO mice (n=7 mice). (F) Representative flow cytometry analysis traces of splenocytes showing the distribution of CD3-positive T cells. (G) Bar graph for the numbers of T cells in control and DSS-treated WT and KO mice (n=5 mice). (H) Representative FACS plot of splenocytes showing the distribution of CD4- and CD8-positive T cells. (I) Bar graph for the numbers of CD4- and CD8-positive T cells in control and DSS-treated WT and KO mice (n=5 mice). Quantification of Western blot data was completed with relative densitometry and normalization to the level of α-tubulin. Data are presented as the means±SEM. Representative data from 3 independent experiments are shown.
**p<0.01, ***p<0.001 compared with the DSS-treated group; #p<0.05, ##p<0.01, ###p<0.001.
|
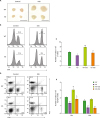 | Figure 4Changes in the T lymphocyte population in mesenteric lymph nodes of T cell-specific STAT3 KO mice. (A) Representative images of mesenteric lymph nodes from control and DSS-treated WT and KO mice. Scale bar=5 mm. (B) Representative flow cytometry analysis traces of lymph node cells showing the distribution of CD3-positive T cells. (C) Bar graph for the numbers of T cells from lymph node cells in control and DSS-treated WT and KO mice (n=5 mice). (D) Representative FACS plot of lymph node cells showing the distribution of CD4- and CD8-positive T cells. (E) Bar graph for the numbers of CD4- and CD8-positive T cells from lymph node cells in control and DSS-treated WT and KO mice (n=5 mice). Data are presented as the means±SEM.
*p<0.05, **p<0.01, ***p<0.001 compared with the DSS-treated group; #p<0.05, ##p<0.01, ###p<0.001.
|
T cell-specific STAT3 deficiency modulates the production of inflammatory cytokines
 | Figure 5T cell-specific STAT3 deficiency affects the production of inflammatory cytokines. (A) Representative FACS plot of a multiplex bead-based immunoassay showing the distribution of cytokine production. (B) Bar graphs for the amounts of IFN-γ, IL-6, IL-10, and IL-17A in the serum from control and DSS-treated WT and KO mice (n=5). (C, D) Bar graphs for the mRNA levels of IFN-γ, IL-6, IL-10, IL-17A, and Foxp3 in colon tissues from control and DSS-treated WT and KO mice (n=5). Relative mRNA levels were normalized to those of GAPDH. Data are presented as the means±SEM. Representative data from 3 independent experiments are shown.
*p<0.05, **p<0.01, ***p<0.001.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download