1. Favre M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J Virol. 1975; 15:1239–1247.

2. zur Hausen H. Viruses in human cancers. Science. 1991; 254:1167–1173.

3. Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005; 14:1157–1164.

4. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003; 16:1–17.

5. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003; 348:518–527.

6. Greer CE, Wheeler CM, Ladner MB, Beutner K, Coyne MY, Liang H, Langenberg A, Yen TS, Ralston R. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995; 33:2058–2063.

7. Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003; 89:101–105.

8. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004; 364:1757–1765.

9. Volpers C, Schirmacher P, Streeck RE, Sapp M. Assembly of the major and the minor capsid protein of human papillomavirus type 33 into virus-like particles and tubular structures in insect cells. Virology. 1994; 200:504–512.

10. Schiller JT. Papillomavirus-like particle vaccines for cervical cancer. Mol Med Today. 1999; 5:209–215.

11. Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992; 89:12180–12184.

12. Roden RB, Greenstone HL, Kirnbauer R, Booy FP, Jessie J, Lowy DR, Schiller JT.
In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996; 70:5875–5883.

13. Unckell F, Streeck RE, Sapp M. Generation and neutralization of pseudovirions of human papillomavirus type 33. J Virol. 1997; 71:2934–2939.

14. White WI, Wilson SD, Bonnez W, Rose RC, Koenig S, Suzich JA.
In vitro infection and type-restricted antibody-mediated neutralization of authentic human papillomavirus type 16. J Virol. 1998; 72:959–964.

15. Doorbar J, Gallimore PH. Identification of proteins encoded by the L1 and L2 open reading frames of human papillomavirus 1a. J Virol. 1987; 61:2793–2799.

16. Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991; 185:251–257.

17. Rose RC, Bonnez W, Reichman RC, Garcea RL. Expression of human papillomavirus type 11 L1 protein in insect cells:
in vivo and
in vitro assembly of viruslike particles. J Virol. 1993; 67:1936–1944.

18. Sasagawa T, Pushko P, Steers G, Gschmeissner SE, Hajibagheri MA, Finch J, Crawford L, Tommasino M. Synthesis and assembly of virus-like particles of human papillomaviruses type 6 and type 16 in fission yeast Schizosaccharomyces pombe. Virology. 1995; 206:126–135.

19. Nardelli-Haefliger D, Roden RB, Benyacoub J, Sahli R, Kraehenbuhl JP, Schiller JT, Lachat P, Potts A, De Grandi P. Human papillomavirus type 16 virus-like particles expressed in attenuated
Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun. 1997; 65:3328–3336.

20. Cho HJ, Shin HJ, Han IK, Jung WW, Kim YB, Sul D, Oh YK. Induction of mucosal and systemic immune responses following oral immunization of mice with
Lactococcus lactis expressing human papillomavirus type 16 L1. Vaccine. 2007; 25:8049–8057.

21. Palker TJ, Monteiro JM, Martin MM, Kakareka C, Smith JF, Cook JC, Joyce JG, Jansen KU. Antibody, cytokine and cytotoxic T lymphocyte responses in chimpanzees immunized with human papillomavirus virus-like particles. Vaccine. 2001; 19:3733–3743.

22. Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005; 12:959–969.

23. Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst. 1994; 86:494–499.

24. Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003; 10:108–115.

25. Christensen ND, Dillner J, Eklund C, Carter JJ, Wipf GC, Reed CA, Cladel NM, Galloway DA. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology. 1996; 223:174–184.

26. Christensen ND, Reed CA, Cladel NM, Hall K, Leiserowitz GS. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6. Virology. 1996; 224:477–486.

27. Sehr P, Müller M, Höpfl R, Widschwendter A, Pawlita M. HPV antibody detection by ELISA with capsid protein L1 fused to glutathione
S-transferase. J Virol Methods. 2002; 106:61–70.

28. Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006; 367:1247–1255.

29. Zhang W, Carmichael J, Ferguson J, Inglis S, Ashrafian H, Stanley M. Expression of human papillomavirus type 16 L1 protein in
Escherichia coli: denaturation, renaturation, and self-assembly of virus-like particles
in vitro
. Virology. 1998; 243:423–431.

30. Kim E, Jhun H, Kim J, Park U, Jo S, Kwak A, Kim S, Nguyen TT, Kang Y, Choi I, et al. Species specific antiviral activity of porcine interferon-α8 (IFNα8). Immune Netw. 2017; 17:424–436.

31. Kwak A, Lee Y, Kim H, Kim S. Intracellular interleukin (IL)-1 family cytokine processing enzyme. Arch Pharm Res. 2016; 39:1556–1564.

32. Lee S, Choi DK, Kwak A, Kim S, Nguyen TT, Gil G, Kim E, Yoo KH, Kim IA, Lee Y, et al. IL-32-induced inflammatory cytokines are selectively suppressed by α1-antitrypsin in mouse bone marrow cells. Immune Netw. 2017; 17:116–120.

33. Lee S, Kim E, Jhun H, Hong J, Kwak A, Jo S, Bae S, Lee J, Kim B, Lee J, et al. Proinsulin shares a motif with interleukin-1α (IL-1α) and induces inflammatory cytokine via interleukin-1 receptor 1. J Biol Chem. 2016; 291:14620–14627.

34. Kim H, Gil G, Lee S, Kwak A, Jo S, Kim E, Nguyen TT, Kim S, Jhun H, Kim S, et al. Cytokine-like activity of liver type fatty acid binding protein (L-FABP) inducing inflammatory cytokine interleukin-6. Immune Netw. 2016; 16:296–304.

35. Jo S, Kim E, Kwak A, Lee J, Hong J, Lee J, Youn S, Bae S, Kim B, Ryoo S, et al. Reconstitution of ST2 (IL-1R4) specific for IL-33 activity; no suppression by IL-1Ra though a common chain IL-1R3 (IL-1RAcP) shared with IL-1. Cytokine. 2016; 83:33–40.

36. Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, Dinarello CA. The interleukin-1α precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front Immunol. 2013; 4:391.

37. Hong SN, Jo S, Jang JH, Choi J, Kim S, Ahn SY, Kim JH, Choe WH, Lee SY, Sung IK, et al. Clinical characteristics and the expression profiles of inflammatory cytokines/cytokine regulatory factors in asymptomatic patients with nodular gastritis. Dig Dis Sci. 2012; 57:1486–1495.

38. Bae S, Kang T, Hong J, Lee S, Choi J, Jhun H, Kwak A, Hong K, Kim E, Jo S, et al. The contradictory functions (activation/termination) of neutrophil proteinase 3 (PR3) in IL-33 biological activity. J Biol Chem. 2012; 287:8205–8213.

39. Ma Z, Chen B, Zhang F, Yu M, Liu T, Liu L. Increasing the expression levels of papillomavirus major capsid protein in
Escherichia coli by N-terminal deletion. Protein Expr Purif. 2007; 56:72–79.

40. Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. Multiplex human papillomavirus serology based on
in situ-purified glutathione
S-transferase fusion proteins. Clin Chem. 2005; 51:1845–1853.

41. Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008; 4:425–434.

42. Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009; 5:705–719.

43. Hernandez BY, Ton T, Shvetsov YB, Goodman MT, Zhu X. Human papillomavirus (HPV) L1 and L1-L2 virus-like particle-based multiplex assays for measurement of HPV virion antibodies. Clin Vaccine Immunol. 2012; 19:1348–1352.




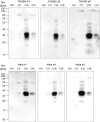
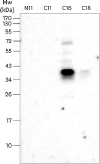




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download