Abbreviations
2-D
2-DE
Akt
BCR
GSK3β
hnRNP
LPS
MAP
MHC
p
PDK1
PI3K
RQ
INTRODUCTION
MATERIALS AND METHODS
Chemicals and laboratory wares
Cell culture
Immunoprecipitation
Two-dimensional electrophoresis (2-DE)
Mass spectrometry
siRNA transfection and sample preparation
RNA isolation and quantitative real-time PCR analysis
Western blot analysis
Statistical analysis
RESULTS
The recruitment of hnRNP A2B1 is inhibited by anti-MHC class II Ab treatment in LPS-treated 38B9 cells
 | Figure 1Identification of hnRNP A2B1 as an MHC class II-associated protein in 38B9 cells. (A) Partial 2-DE image of hnRNP A2B1 (upper panel) and the RT-PCR product of hnRNP A2B1 mRNA (lower panel) prepared from 38B9 cells in the presence or absence of LPS and anti-MHC class II Ab. MHC class II-associated proteins were prepared by immunoprecipitation from whole-cell lysates using an anti-MHC class II Ab and separated by 2-DE as described in the Materials and Methods section. GAPDH was used as an internal loading control for RT-PCR. Molecular mass and isoelectric point of the candidate protein included in the spot were calculated as 37.4 kDa and 8.97, respectively, using AxPASy inline tool and candidate protein was expected to be hnRNP A2B1 based on mass analysis data. (B) Western blot analysis of hnRNP A2B1 protein present in 38B9 cells after the indicated treatments. Actin was used as an internal loading control. (C) Whole-cell lysates from 38B9 cells were immunoprecipitated using the anti-MHC class II Ab after the indicated treatments and immunoblotted using the hnRNP A2B1 Ab. The numbers under each gel picture represent the RQ. Representative data from at least 3 independent experiments are presented. |
hnRNP A2B1 is associated with MAP kinase signaling in 38B9 cells
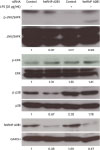 | Figure 2Effect of hnRNP A2B1 on the activation of components of the MAP kinase signaling pathway in 38B9 cells. The 38B9 cells were transfected with hnRNP A2B1 siRNA. Twenty-four hours after transfection, cells were treated with 25 μg/ml LPS for 30 min at 37°C. Western blot analyses of the levels of total and p-ERK, p38, and JNK/SAPK in 38B9 cells following the indicated treatments after knocking down hnRNP A2B1 as described in the Materials and Methods section. Actin was used as an internal loading control. The numbers under each gel picture represent the RQ. Representative data from at least 3 independent experiments are presented.
SAPK, stress-activated protein kinase.
|
hnRNP A2B1 is involved in the regulation of NF-κB activation
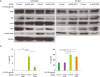 | Figure 3Effect of hnRNP A2B1 siRNA treatment on LPS-mediated NF-κB activation and the expression of pro-inflammatory cytokines in 38B9 cells. (A) The 38B9 cells were transfected with hnRNP A2B1 siRNA. Twenty-four hours after transfection, cells were treated with 25 μg/ml LPS for 30 min at 37°C, and proteins in the cytoplasmic and nuclear fractions were analyzed by western blotting. GAPDH was used as an internal control. Representative data from 2 independent experiments are presented. (B) The 38B9 cells were transfected with hnRNP A2B1 siRNA. Twenty-four hours after transfection, cells were treated with 25 μg/ml LPS for 12 h at 37°C, and cell culture supernatants were harvested. The levels of IL-6 (left panel) and TNF (right panel) in the culture supernatants were analyzed as described in the Materials and Methods section. Data are presented as means±standard deviation of duplicate determinations, and a representative result from 2 independent experiments is shown.
TNF, tumor necrosis factor.
*p<0.05 and **p<0.01 indicate significant differences between groups.
|
hnRNP A2B1 is involved in Akt signaling in 38B9 cells
 | Figure 4Effect of hnRNP A2B1 siRNA treatment on components of the Akt signaling pathway in 38B9 cells. The 38B9 cells were transfected with hnRNP A2B1 siRNA. Twenty-four hours after transfection, cells were treated with 25 μg/ml LPS at 37°C for 30 min. Western blot analyses of the expression and activation of Akt, GSK3β, and Mcl-1 were performed in cells after the indicated treatments. Actin was used as an internal loading control for western blot analyses. The numbers under each gel picture represent the RQ. Representative data from at least 3 independent experiments are presented. |
hnRNP A2B1 is involved in B cell maturation
 | Figure 5Effect of hnRNP A2B1 silencing on the differentiation of LPS-treated 38B9 cells. The 38B9 cells were transfected with hnRNP A2B1 siRNA. Twenty-four hours after transfection, cells were treated with 25 μg/ml LPS at 37°C for 12 h. Expression of the germline Igκ gene in 38B9 cells after the indicated treatments was determined by (A) RT-PCR and (B) quantitative real-time PCR. In addition, the level of surface IgM expression was determined by flow cytometry. Representative data from at least 3 independent experiments are shown.
*p<0.05 and **p<0.01 indicate significant differences between groups.
|
DISCUSSION
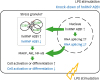 | Figure 6Schematic diagram outlining possible regulatory mechanism of cellular responses by hnRNP A2B1 in LPS-stimulated 38B9 cells. LPS stimulation of 38B9 cells increases the level of hnRNP A2B1 protein and may accumulate in stress granule in cytoplasm, which, in turn, activates cellular signaling pathways to activate 38B9 cells. These signaling pathways are suppressed by knocking-down of hnRNP A2B1. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download