INTRODUCTION
MATERIALS AND METHODS
Cells
Reagents
NO assay
MTT assay
Cell cycle analysis
Apoptosis/necrosis detection
RESULTS
PVDF treatment decreased NO production in LPS-activated macrophages
 | Figure 1Effect of PVDF membrane on NO production in LPS-stimulated RAW cells. RAW cells were cultured and stimulated with PBS or LPS. These cells were then treated with one of the membranes for 24 h, as indicated. Cells treated with DMEM served as negative controls. Culture supernatants were harvested and tested for NO production. Data are shown as mean±standard deviation values of triplicates in each group. These data are representative of 2 independent experiments.
***p<0.001.
|
The number of macrophages was reduced following treatment with CNT-attached PVDF
 | Figure 2Influence of PVDF membrane on RAW cell survival with or without LPS stimulation. PBS or LPS-stimulated RAW cells were treated with the indicated membrane for 24 h. DMEM treated RAW cells were used as negative controls. After removing the culture supernatants, the cells were examined for survival compared with the negative controls. The plotted values represent survival rate±standard deviation. These data are representative of 2 independent experiments.
***p<0.001.
|
PVDF increased cell cycle of macrophages
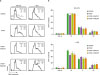 | Figure 3Altered cell cycle in RAW cells after exposure to PVDF membrane. Cells were treated with PVDF, PVDF+SWCNT, or PVDF+MWCNT. The cells were then exposed to LPS or LPS-free conditions for 21 h, and then stained using the Krishan staining buffer. (A) Stained cells were analyzed by flow cytometry and a histogram was obtained. The cell cycle of these cells was examined based on the DNA content of RAW cells. (B) The plotted values represent mean±standard deviation. The data are representative of 3 independent experiments.
*p<0.05.
|
PVDF treatment induced apoptosis in murine macrophages
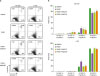 | Figure 4Increased apoptosis in RAW cells after treatment with PVDF. Cells were treated with the 3 types of membrane: PVDF, PVDF+SWCNT, and PVDF+MWCNT. The cells were then exposed to LPS or LPS-free conditions for 21 h and then stained with annexin V and propidium iodide. (A) Stained cells were examined by flow cytometry. (B) The data shown are means of triplicates±standard deviation. Similar results were obtained in 3 independent experiments.
*p<0.05.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download