Abbreviations
A/PR8
AM
BAL
BW
DC
GzB+
IFN
IP-10
KC
MCP-1
MHC
MPL
PenH
RANTES
SEM
sPR8
Th1
TLR
TNF
INTRODUCTION
MATERIALS AND METHODS
Animals and reagents
In vitro stimulation assays of DCs
Immunization and virus infection
ELISA for antibody and cytokine levels
Lung virus titration
Flow cytometry
Statistical analysis
RESULTS
Combination of MPL and CpG enhances DC activation and cytokine production
 | Figure 1
In vitro activation of bone marrow-derived DCs by adjuvant stimulation. DCs were enriched from mouse bone marrow cells by treatment with mGM-CSF. (A-C) Cytokine levels secreted into the culture supernatants of DCs treated with different concentrations of MPL and CpG were measured by ELISA. For statistical analysis, Two-way ANOVA and Bonferroni post-multiple comparison tests were performed. (D-F) The immature DCs were cultured with MPL (0.2 μg/ml), CpG (1 μg/ml), or MPL (0.2 μg/ml)+CpG (1 μg/ml) for 2 days. Expression levels of DC activation markers were determined by flow cytometry. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
mGM-CSF, mouse granulocyte-macrophage colony stimulating factor.
*p<0.033; **p<0.002; ***p<0.001 between the indicated groups.
|
DCs with combination MPL and CpG treatment are effective in activating T cells
 | Figure 2
In vitro proliferation and activation of T cells by adjuvant-activated DCs. DCs enriched from bone marrow cells were pre-activated by MPL (0.2 μg/ml), CpG (1 μg/ml), or MPL (0.2 μg/ml)+CpG (1 μg/ml) for 2 days. Allogeneic lymphocytes were harvested from spleens of C57BL/6 mice. CFSE-labeled lymphocytes and pre-activated DCs were co-cultured for 5 days. T cell proliferation and cytokine producing cells were determined by flow cytometry. (A) Proliferated CD4+ T cells. (B) Proliferated CD8+ T cells. (C) IFN-γ producing CD4+ T cells. (D) IFN-γ producing CD8+ T cells. (E) IL-4 producing CD4+ T cells. (F) IL-4 producing CD8+ T cells. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
ns, not significant between the indicated groups.
*p<0.033; **p<0.002; ***p<0.001.
|
Combination MPL and CpG adjuvants enhance IgG antibody responses to influenza vaccine
 | Figure 3TLR agonist adjuvant effects on inducing IgG antibodies specific for influenza vaccine antigen. BALB/c mice (n=5) were immunized with sPR8 virus vaccine only or sPR8 virus vaccine in the presence of TLR agonist adjuvants (MPL, CpG, or MPL+CpG). Immune sera were taken 2 weeks after immunization and PR8 virus antigen-specific IgG antibody levels were measured by ELISA. IgG (A), IgG1 (B) and IgG2a (C) levels were shown in OD values at 450 nm. (D) IgG2a/IgG1 ratio was calculated at 102 times sera dilution. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
*p<0.033; **p<0.002; ***p<0.001 between the indicated groups or compared to sPR8 group; †p<0.033 compared to sPR8+MPL.
|
Both MPL and CpG adjuvants enhance protective efficacy of influenza vaccination
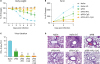 | Figure 4MPL and CpG adjuvant effects on improving protective efficacy of influenza vaccination after lethal virus infection. The immunized mice (n=5) were infected with A/PR8 virus (2×LD50) after 6 weeks of immunization. BW (A) and PenH (B) were measured for 7 days after infection and % changes were calculated based on the day 0. (C) Lung samples were harvested day 7 post infection. Lung virus titers of each immunized mice were measured by using embryonated eggs. EID50 were shown. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed. (D) Lung histopathology. Intact lungs were harvested at day 7 post infection, fixed, processed, and stained with hematoxylin and eosin.
EID50, 50% egg infection dose; inf., infection.
***p<0.001 compared to naïve infection group; †††p<0.001 compared to sPR8 group.
|
Combination MPL and CpG adjuvanted vaccination prevents inflammatory innate immune responses due to virus infection
 | Figure 5Cytokines and chemokines in lung samples after lethal virus infection of mice. Lung samples were harvested from the immunized mice (n=5) day 7 post A/PR8 virus infection. Cytokine and chemokine levels of each lung samples were measured by ELISA. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison test were performed.
*p<0.033; **p<0.002; ***p<0.001 compared to Naïve infection group; †p<0.033; ††p<0.002; †††p<0.001 compared to sPR8 group.
|
 | Figure 6Cellular infiltration in lungs after lethal virus infection. The immunized mice (n=5) were infected with A/PR8 virus (2×LD50) after 6 weeks of immunization. Lung samples were harvested day 7 post infection, and cell phenotypes were determined by flow cytometry and calculated by multiplying cell percentages with total cell numbers. (A) Total lung cells. (B) AMs; CD11b−CD11c+F4/80+. (C) Monocytes; CD11b+F4/80+Ly6Chigh. (D) Neutrophils; CD11b+F4/80−Ly6c+. (E) pDCs; CD45+F4/80−CD11c+MHCIIhighB220+. (F) CD103 + DC; CD45+F4/80−CD11c+MHCIIhighCD11b−CD103+. (G) cDC; CD45+F4/80−CD11c+MHCIIhighCD11b+. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
pDC, plasmacytoid dendritic cell; cDC, conventional DC.
*p<0.033; **p<0.002 compared to naïve infection group; †p<0.033 compared to sPR8 group.
|
MPL and CpG combination adjuvanted vaccination does not induce inflammatory T cells due to influenza virus infection in the airways and lungs
 | Figure 7Cytokine producing T cells after immunization and lethal virus infection. The immunized mice were infected with a lethal dose (2×LD50) of A/PR8 virus after 6 weeks of immunization. Lung and BAL samples were harvested day 7 post infection. Intracellular cytokine staining was performed after incubation with MHCI and II-restricted peptides for CD8 and CD4 T cell stimulation as described in the Materials and Methods section. The cytokine producing cell numbers were calculated by multiplying cell percentages with total cell numbers. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
*p<0.033; **p<0.002 compared to naïve infection group; †p<0.033 compared to sPR8 group.
|
MPL and CpG adjuvanted vaccination enhances antigen-specific cytokine producing splenocytes
 | Figure 8Cytokine production of spleen cells from the immunized mice after in vitro antigen stimulation. Spleen cells were harvested from the immunized mice day 7 post infection and then cultured with inactivated A/PR8 virus stimulation. After 3 days culture, cytokine levels in supernatants were determined by ELISA. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
nd, not detected or values below detection limit.
**p<0.002; ***p<0.001 compared to naïve infection group; ††p<0.002; †††p<0.001 compared to sPR8 group.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download