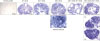
The localization of SAA3 mRNA in the whole ovary was performed in mouse ovulation model using
in situ hybridization as described previously (
720). Ovaries were collected at post-eCG injection (0, 24, and 48 h) and post-hCG (2 and 24 h) (
Fig. 1). First, ovarian CYP19 (aromatase) mRNA was localized to characterize and validate the mouse ovulation model (
Fig. 1). CYP19 mRNAs are expressed in the granulosa layer of large follicles during follicular development, with maximal expression occurring immediately after ovulation and loss of expression with luteinization (
Fig. 1) as anticipated (
2425). The expression pattern of ovarian CYP19 mRNA indicates that the mouse ovulation model reflects the physiological process of follicular development and ovulation. Next, we determined the localization of SAA3 mRNA expression in the whole ovaries using the mouse ovulation model. We confirmed no signal with sense SAA3 probe (
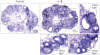
Fig. 2). SAA3 mRNA is lightly and broadly expressed throughout the whole ovary even prior to eCG injection (
Fig. 2). During follicle development, granulosa cells express higher SAA3 mRNA than thecal-interstitial cells, and small follicles express higher SAA3 mRNA than large follicles (
Fig. 2). Interestingly, SAA3 mRNA is intensely expressed in ovarian follicle atresia (
Fig. 2) in which immature ovarian follicles are degenerated by granulosa cell apoptosis. Because atresia in ovarian follicles involves atretogenic factors such as TNF-α and Fas ligands (
26), these factors are likely to induce SAA3 mRNA which is strictly regulated by NF-κB signaling (
715). At 72 h ovary (48 h post-eCG and 24 h post-hCG injections), corpora lutea (CL) contain cells with intense SAA3 mRNA hydridization (
Fig. 2). Because TNF-α is a product of luteal macrophages that infiltrate into the CL (
27), immune cell-derived proinflammatory cytokines are likely to induce SAA3 during CL formation and luteal regression. NF-κB mediated signaling is primary pathway inducing SAA3 expression in mouse granulosa cells (
715). Therefore, we employed treatment with IL-1β a proinflammatory cytokine activating NF-κB signaling, and assessed localization of SAA3 mRNA by
in situ hybridization. Intense SAA3 mRNA hybridization was localized to CL (
Fig. 3) similar to the pattern observed in newly formed CL using the ovulation model (
Fig. 2). IL-1β-treated ovary has more intense and extensive SAA3 mRNA expression compared to non-treated ovary (
Fig. 3). Interestingly, following IL-1b treatment SAA3 mRNA was intensely localized to the thecal-interstitial cell layers in both small and large follicles (
Fig. 3). These findings indicate that ovarian follicles likely protect their oocytes by blocking harmful effects due to inflammatory stimulation. Direct effects of proinflammatory cytokines on SAA3 expression were then assessed using cultured primary granulosa cells.
In vitro treatment with TNF-α, IL-1α, or IL-1β strongly induces SAA3 mRNA expression in mouse granulosa cells compared to non-treated granulosa cells (
Fig. 4A). Granulosa cell-specific expression of SAA3 was further assessed by collection of granulosa throughout the induction of follicle development and ovulation in the mouse ovulation model. Granulosa cells collected at 48 h post-eCG plus 24 h post-hCG injection highly express SAA3 mRNA (
Fig. 4B), reflecting CL derived SAA3 expression as indicated in
Fig. 2. On the other hand, granulosa cells collected prior to this time point expressed modest SAA3 documented by only a faint band following RT-PCR. SAA3 expression in immature granulosa cells could be stimulated by LPS, a strong inflammatory mediator. In another set of experiments mice were injected intraperitoneally with LPS and then treated with gonadotropins as the ovulation model with granulosa cells collected at various time points. Granulosa cells collected following injection with LPS and prior to eCG injection express increase SAA3 mRNA compared to mice not receiving LPS (
Fig. 4B). In addition, SAA3 mRNA expression was further increased in cells collected at the 48 h post-eCG plus 24 h post-hCG time point in LPS treated mice compared to mice not receiving LPS (
Fig. 4B). SAA expression in mouse ovaries was further assessed by examination of 2 different GEO data sets. Each data set indicates that SAA3 is the primary SAA isoform in the ovary (
Fig. 4C and 4D). Furthermore, SAA3 expression was increased following eCG plus 24 h post hCG (
Fig. 4C) similar to the findings of the current study (
Fig. 4B). Additional data indicate that SAA3 expression in granulosa cells comprising the cumulus-oocyte-complex is increased following eCG plus 16 h post hCG (
Fig. 4D). Together these 2 GEO datasets support the findings of the current study on high expression of SAA3 in CL as shown in
Fig. 2.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download