INTRODUCTION
MATERIALS AND METHODS
Study subjects
Construction of synthetic peptides
Isolation of 127-Tregs and 45RA-Tregs
Stimulation of 127-Tregs and 45RA-Tregs with Pep19-pulsed DCs in a tolerogenic milieu
Stimulation of 127-Tregs and 45RA-Tregs with Pep19-pulsed DCs
Ex vivo expansion of Tregs
Phenotypic, functional, and epigenetic analysis of the expanded Tregs
Evaluation of Treg phenotypic markers
A carboxyfluorescein diacetate succinimidyl ester (CFSE)-based in vitro suppression assay to bystander antigens
Treg proliferation to Pep19 and bystander antigens
Expression of the homing receptors CCR7 and CD62L
Evaluation of CD95L-mediated apoptosis
Epigenetic analysis of the Treg-specific demethylation region (TSDR)
Adoptive transfer of human Tregs into NOD/scid/IL-2Rγ−/− mice
Induction of collagen-induced arthritis
Evaluation of the in vivo suppression of arthritis
Expression of Treg markers by splenic Tregs
RESULTS
The number of ex vivo expanded Tregs
Table 1
Initial numbers, ex vivo expanded numbers, and fold increase of each Treg subpopulation from study subjects

Phenotypic and functional analysis of the expanded Tregs
Treg phenotypic markers in the expanded Tregs
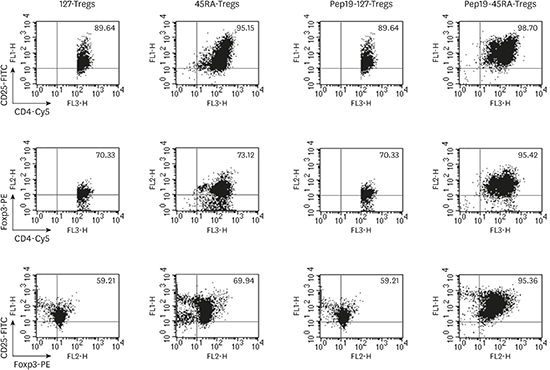
 | Figure 1Fluorescence-activated cell sorting profiles of each representative subject demonstrating the expression of suppressive Treg markers — CD4, CD25, and Foxp3 — in ex vivo expanded 127-Tregs, 45RA-Tregs, Pep19-127-Tregs, and Pep19-45RA-Tregs. While a similar staining intensity was observed among 127-Tregs, 45RA-Tregs, and Pep19-127-Tregs, the highest intensity of these 3 phenotype markers was seen in Pep19-45RA-Tregs.
Treg: regulatory T cell, Pep19: peptide 19, 127-Tregs: CD4+CD25+CD127lo− regulatory T cells, 45RA-Tregs: CD4+CD25+CD45RA+ regulatory T cells, Cy5: cyanine 5.
|
Expression of the homing receptors CCR7 and CD62L
 | Figure 2Fluorescence-activated cell sorting profiles of CD62L and CCR7 expression in the expanded Tregs of each representative subject. While 45RA-Tregs and Pep19-45RA-Tregs demonstrated a modest level of expression of CD62L and CCR7, 127-Tregs and Pep19-127-Tregs demonstrated a higher level of expression of these markers.
Treg: regulatory T cell, 127-Tregs: CD4+CD25+CD127lo− regulatory T cells, Pep19: peptide 19, 45RA-Tregs: CD4+CD25+CD45RA+ regulatory T cells, Cy5: cyanine 5.
|
The CFSE-based in vitro suppression assay
 | Figure 3(A) Fluorescence-activated cell sorting profiles demonstrating the suppressive capability of expanded 127-Tregs, 45RA-Tregs, Pep19-127-Tregs, and Pep19-45RA-Tregs from each representative subject on the Teff proliferative response to Pep19 and the bystander self-antigens Hu19, Hu9, Hu14, and ox-LDL, as analyzed by the CFSE assay. (B) Bar graph demonstration of the mean±standard deviation of the fluorescence-activated cell sorting profiles of all subjects. Suppression of the Teff proliferative response to Pep19 and the various bystander self-antigens was most evident in Pep19-45RA-Tregs.
127-Tregs: CD4+CD25+CD127lo− regulatory T cells, 45RA-Tregs: CD4+CD25+CD45RA+ regulatory T cells, Pep19: peptide 19, CFSE: carboxyfluorescein diacetate succinimidyl ester, Hu: human, ox-LDL: oxidized low-density lipoprotein, Teff: effector T cell.
|
Proliferation of the expanded Tregs in response to Pep19 and various bystander self-antigens
 | Figure 4(A) Fluorescence-activated cell sorting profiles of expanded 127-Tregs, 45RA-Tregs, Pep19-127-Tregs, and Pep19-45RA-Tregs from each representative subject demonstrating a robust proliferative responses to Pep19 and the bystander self-antigens Hu19, Hu9, Hu14, and ox-LDL. Bar graph demonstration of the mean±standard deviation of the fluorescence-activated cell sorting profiles of all subjects. (B) The proliferative response of Pep19-45RA-Tregs was more pronounced than that of the 3 other kinds of Tregs.
127-Tregs: CD4+CD25+CD127lo− regulatory T cells, 45RA-Tregs: CD4+CD25+CD45RA+ regulatory T cells, Pep19: peptide 19, Treg: regulatory T cell, CFSE: carboxyfluorescein diacetate succinimidyl ester, Hu: human, ox-LDL: oxidized low-density lipoprotein.
|
CD95L-mediated apoptosis
 | Figure 5Fluorescence-activated cell sorting profiles of CD95-mediated apoptosis in expanded Tregs of a representative subject after 24 hours of incubation. While 127-Tregs and Pep19-127-Tregs demonstrated a similar resistance to CD95L-mediated apoptosis, 45RA-Tregs and Pep19-45RA-Tregs exhibited slightly greater resistance.
Treg: regulatory T cell, 127-Tregs: CD4+CD25+CD127lo− regulatory T cells, Pep19: peptide 19, 45RA-Tregs: CD4+CD25+CD45RA+ regulatory T cells, PI: propidium iodide, PE: phycoerythrin, FITC: fluorescein isothiocyanate.
|
Epigenetic analysis of the TSDR of the expanded Tregs
 | Figure 6Bar graph representation of the percentage of expanded Tregs in a representative subject expressing the Foxp3 protein and the percent of DNA demethylation in the Foxp3 TSDR. While a similar degree of DNA demethylation in the Foxp3 TSDR was among the Treg groups, the intensity of Foxp3 expression was most pronounced in Pep19-45RA-Tregs.
Treg: regulatory T cell, TSDR: Treg-specific demethylation region, Pep19: peptide 19, 127-Tregs: CD4+CD25+CD127lo− regulatory T cells, 45RA-Tregs: CD4+CD25+CD45RA+ regulatory T cells.
Statistically significant value, a)P<0.01; b)P<0.001.
|
In vivo suppression of arthritis in humanized mice
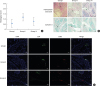 | Figure 7(A) Bar graph representation of the severity of arthritis score of each mouse group on a scale of 0 to 3. (B) The lowest score was seen in the group III mice, which were infused with Pep19-45RA-Tregs. Representative micrographs of articular joints and cartilage stained with hematoxylin and eosin and safranin-O staining of articular cartilage in mice from groups I–III (bar=10 µm). (C) No evidence of proteoglycan loss from articular cartilage (a marker of cartilage destruction) was observed in the mice in groups I and III. The group II mice demonstrated a variable degree of proteoglycan loss from the articular cartilage. Representative micrographs of the immunohistochemical localization of CD4+CD25+ Tregs in articular joints in groups I–III. The homing pattern of CD4+CD25+ Tregs to the site of inflammation was most pronounced in the articular joints in group III (bar=50 µm).
Pep19: peptide 19, 45RA-Tregs: CD4+CD25+CD45RA+ regulatory T cells, Treg: regulatory T cell.
|
 | Figure 8(A) Photographic features demonstrating swollen footpads in a representative mouse from each group. (B) Line graphs depicting the changes in the mean thickness of the swollen footpad at each observation period. (C) Bar graph representing the mean paw thickness (±standard deviation) of each mouse group on day 38. Mice from both groups II and III showed minimal thickness, while the group I mice demonstrated greatest thickness. The value was significantly smaller in group III when compared with that of group I. (D) A diagrammatic plot of the mean survival rate of each mouse group during the observation period. The highest survival rate was seen in the group III mice.
a)Statistically significant value (P<0.05).
|
Expression of Treg markers by splenic Tregs
 | Figure 9(A) Expression of the Treg markers CD25 and Foxp3 by splenic CD4+ T cells isolated from a representative mouse from each group (I–III) in response to HSP60 from P. gingivalis, Pep19, Hu19, Hu9, Hu14, and ox-LDL as analyzed by fluorescence-activated cell sorting. (B) These Treg markers were variably expressed in response to the tested antigens in all 3 groups. Bar graph representation of the fluorescence-activated cell sorting profiles of CD25+Foxp3+ splenic Tregs in response to various bystander antigens. Statistically significant differences were noted at variable levels among the test groups except for the cognate epitope spreader antigen Pep19.
Treg: regulatory T cell, Pep19: peptide 19, Hu19: peptide 19 of human HSP60, Hu9: peptide 9 of human HSP60, Hu14: peptide 14 of human HSP60, ox-LDL: oxidized low-density lipoprotein.
Statistically significant value, a)P<0.05; b)P<0.01; c)P<0.001.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download