INTRODUCTION
A prerequisite for successful periodontal and implant surgery is tension-free primary closure of soft tissue [
1]. Oftentimes, because soft tissues follow the underlying bony contour [
2], severe alveolar bone resorption is accompanied by a limited amount of soft tissue, which impairs tension-free primary closure.
Accumulated evidence suggests that an increase in soft tissue volume overlying the bone predictably results from distraction osteogenesis [
34]; however, in clinical reality, this procedure is complex and technically demanding [
5]. An elegant alternative, which has demonstrated promising results, is to create a surplus of soft tissue by expanding the preexisting soft tissue layer prior to bone augmentation and placement of endosseous implants [
6]. The growing need for expansion of soft tissue in the oral area led to the development of new expanders. Today, soft tissue expanders (STEs) consist of a hydrogel-based core coated with a silicone-perforated membrane. This outer shell characterizes second-generation STEs, in contrast to the first-generation devices without such a membrane, and allows self-inflating osmotic expansion [
7].
Most previous studies evaluating the healing of second-generation STEs in the oral area have investigated outcomes following oral soft tissue expansion in small animals [
89]. Investigations in dogs or humans have been limited to results following bone augmentation [
61011]. While the tissue reaction to STE is believed to have a major impact on soft tissue quality after expansion, little is known about the characteristics of the bone and soft tissues surrounding STE in an animal setting that more closely mimics the healing events in humans. The existent data are conflicting, as both reactive soft tissue encapsulation of STE and bone resorption have been reported [
91213]. In addition, no correlation has yet been established between the properties of the devices and tissue reactions
in vivo.
In the present study, STEs with 3 different volumes were first investigated in vitro to determine their swelling and mechanical properties. Subsequently, the in vivo outcomes in terms of soft tissue expansion were experimentally verified using a canine model. The aim of this study was to test whether the in vitro properties of these STEs were compatible with an in vivo tissue reaction allowing expansion of soft tissue with minimal remodeling of the underlying alveolar bone.
MATERIALS AND METHODS
STEs
The STEs employed in the present study consisted of second-generation self-inflating osmotic expanders incorporating 2 parts: 1) a hydrogel core, made from methyl-methacrylate with the property of osmotically absorbing body fluids and becoming infiltrated; and 2) a N-vinyl pyrrolidone shell surrounding the core, allowing body fluids to flow through perforations with a radius of 0.5 mm (
Figure 1). Before animal experiments were conducted, 3 STEs (
S,
M,
L) with different volumes (0.15, 0.25, and 0.42 mL, respectively) in a silicone envelope were first investigated to determine their swelling characteristics and mechanical properties. The STEs and surgical instruments used for implanting the tissue expanders were provided by Osstem (Busan, Korea).
 | Figure 1Unswollen self-infiltrating osmotic expander used in the study, enveloped in its silicone shell.
|
Mass and volumetric swelling behavior
For each volume of the STEs (0.15, 0.25, and 0.42 mL), 20 samples were immersed in 5 mL of 0.9% saline solution within an Eppendorf tube and incubated. After 24 hours, 10 samples from each STE type were collected to measure their post-expansion size (length and radius), enabling the amount of expansion to be calculated. For the remaining samples, the test was continued until the equilibrium swelling ratio was obtained on day 28. Then, the hydrogels were taken out from the solution, gently dried with filter paper in order to remove the excess water from their surface, and then weighed (M
post). The mass swelling behavior (S
t) was calculated based on the change in mass over time using the following equation [
14]:
Although the degree of hydrogel expansion is usually measured as the ratio of change in mass over a time interval, volume change is more important than mass variation for tissue expanders [
15]. The volume swelling ratio (V
t) was therefore calculated in a similar way to the mass swelling ratio, as the volume change in the hydrogel at the time of measurement divided by the original volume of the tissue expander [
15]:
Mechanical properties
The mechanical properties of the STEs were measured in their equilibrium-swollen state using an Instron 3365 Universal Testing Machine (Instron Corp., Norwood, MA, USA) (ultimate tensile strength [UTS]). On each STE type (S, M, or L) a force load of 60 N and a crosshead speed of 1 mm/min was employed in compressive and tensile modes. The ultimate compressive load (n=10 for each type), tensile strength (n=5 for each type), and tensile strain (n=5 for each type) were measured.
Animal experiments
In vivo experiments were conducted to evaluate the biocompatibility of the engineered hydrogels and to test their expansion capability. For this purpose, a total of 5 beagle dogs (1 year of age, weighing 10 kg) were employed. All applied protocols were approved by the Institutional Animal Care and Use Committee, Cronex, Hwasung, Korea (CRONEX-IACUC-201406006). Operations were carried out under general anesthesia using an intravenous injection of Zoletil® (0.1 mg/kg, Vibac, Carros, France), Rompun® (2.3 mg/kg, Bayer Korea, Ansan, Korea), and atropine sulfate (0.05 mg/kg, Jeil, Daegu, Korea), in addition to local anesthesia using an infiltration of lidocaine (20 mg/kg, Huons, Seongnam, Korea).
Soft tissue expansion
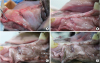
To prepare the alveolar ridges to receive the STEs, the right second, third, and fourth premolar teeth were extracted from the lower jaw of each animal and primary wound closure was obtained using resorbable interrupted sutures. After the resulting alveolar ridges were allowed to heal for 2 months, STEs were implanted. A template was first applied to determine the adapted size of the expander. Then, a 1.5-cm-long vertical incision was made on the buccal alveolar mucosa from the region of the extracted teeth, before a subperiosteal pouch was created by elevating the periosteum using an elevator. The size of the pouch was determined according to the surgical template corresponding to the initial volume of the tissue expander; then, the STE was placed into the pouch, fixed with a screw to the bone, and primary wound closure was obtained using resorbable interrupted sutures (
Figure 2). Dogs were fed a soft diet to avoid any mechanical interference with post-surgical healing. After 4 weeks, euthanasia was performed by injecting an overdose of potassium chloride (Jeil, Seoul, Korea). The jaws were dissected and blocks containing the experimental specimens were obtained. All specimens were fixed in phosphate-buffered saline-buffered formalin (10%) solution (pH=7.4).
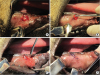 | Figure 2(A) A vertical incision measuring 1.5 cm in length was performed in the buccal mucosa of the edentulous area and a subperiosteal pocket was prepared using a periosteal elevator. (B) After a subperiosteal pouch was created, the expander was inserted carefully under the periosteum. (C) The expander was fixed by a bone screw on the alveolar bone. (D) Before the vertical incision was sutured.
|
Histologic processing and analysis
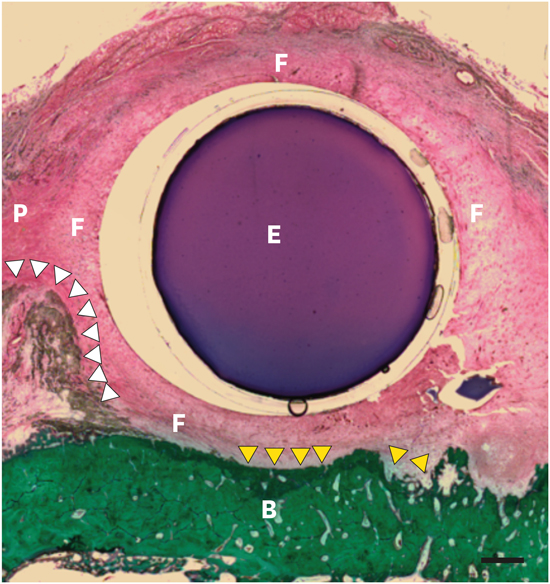
Following fixation, bone core biopsies and jaw blocks containing the fixtures were dehydrated and embedded in light-curing methyl methacrylate (Technovit 7200, Kulzer GmbH, Wehrheim, Germany) before being glued with acrylic cement (Technovit 7210, Kulzer GmbH) to plexiglass slides. Step-serial sections using the grinding technique yielded specimens approximating 30 µm in thickness. The most central aspect of the expander in the buccolingual aspect was used as the section direction. Histological slides were stained with Masson-Goldner trichrome and hematoxylin, and examined by optical microscopy (Olympus, Tokyo, Japan). The images were captured using image analyzing software (Tomoro Scope Eye, Techsan Digital Imaging, Seoul, Korea).
Fibrous capsule thickness for each section was measured as the average thickness at 5 different random locations, and was reported as the average thickness per implant.
Statistical analysis
The mean and standard deviation values of the in vitro (mass swelling behavior, ultimate compressive load, tensile strain, tensile strength) and histomorphometric parameters (fibrous capsule thickness) were calculated. In order to statistically compare the in vitro parameters, analysis of variance with the Tukey test for post hoc analysis was used to quantify differences among the S, M, and L groups for mass swelling behavior and the ultimate compressive load. The Kruskal-Wallis test with the Dunnet test for post hoc analysis was used to compare the differences among the S, M, and L groups for tensile strain and tensile strength. P values <0.05 were considered to indicate statistical significance.
DISCUSSION
A way to palliate soft tissue deficiency, prior to horizontal and vertical reconstruction of the alveolar ridge, is soft tissue expansion using an expandable device to increase the tissue area.
In the present study, a second-generation osmotic expander was first investigated in vitro to determine its mechanical and swelling properties. Subsequently, tissue reactions to expander-induced compression were assessed clinically and histologically 1 month following STE installation on the alveolar ridge in dogs. While the swelling properties remained stable in vivo, significant effects were observed on the underlying bone and surrounding periosteum.
The concept of soft tissue expansion is based on the biological properties of various soft tissues, such as skin or mucous membranes, to react to applied mechanical forces by tissue growth resulting from cell proliferation [
16]. Expanded tissues, therefore, react in accordance to the intrinsic characteristics of the expander. The mechanical properties, as well as swelling and other parameters, are a result of the composition of the hydrogel at the expander's core [
17]. The inclusion of methyl methacrylate in the osmotic hydrogel, similarly to the expander used in this study, results in greater osmotic potential and subsequent amplification of the swelling capability when compared to hydroxyethyl methacrylate [
181920] or non-ionic hydrogels [
20]. The STEs used in the present study were able to expand their initial volumes between 4.7 and 5.2 times in physiological saline solution. Comparably, a volume swelling ratio of 6 times, reported for Osmed
®, a self-inflating osmotic expander, was sufficient to provide an adequate amount of soft tissue after 2 weeks in rabbit mandibles [
9]. From a mechanical point of view, STE properties influence the healing outcomes. For example, devices that are excessively stiff (very high ultimate compressive load) or too soft (very low ultimate compressive load) would not be compatible with a physiological tissue reaction [
21]. Werfully et al. [
22] demonstrated that the force exerted on bone by a full thickness flap following primary closure reached 518 g at day 10 postoperatively and continued to increase over time to exceed 1,733 g at 28 days following surgery. In light of the results of the STE compressive test in this study, it can be inferred that the tissue expander, with a mean ultimate compressive load varying between 1,497.3 and 1,464.6 g, is able to bear the stress from the flap up to 28 days, a time point at which the tissue expander has a high risk of fracture. This is in line with the findings from the
in vivo experiment demonstrating a stable size of the expander at 28 days after insertion. Here, the critical effect of biomechanical interactions is not only limited to the flap/expander interface. When applied subperiosteally, tissue expanders apply expansion force onto the peripheral tissues, including the enveloping soft tissue as well as the underlying alveolar bone. A continuous pressure was demonstrated to induce alveolar bone resorption when a threshold of approximately 70 g/cm
2 was exceeded [
23]. Extrapolated to the tensile strain tested for the STEs in the present study, an inexorable resorptive bone reaction would be expected at the expander/bone interface, as the mean tensile force ranged between 81.3 and 98.3 g/cm
2.
To verify this assumption, STEs were implanted subperiosteally on healed alveolar ridges in dogs using a tunnel approach. Due to the lack of view and freedom of manipulation with the tunnel technique, placement and fixation with this technique is believed to be less easy than the flap procedure [
24]. Nevertheless, tunnelization is still recommended for STE insertion [
7], given the need for a less invasive incision that would result in a smaller scar and a reduced risk of wound dehiscence [
25]. A study comparing the flap and tunnel approaches in the maxilla in a goat model failed to show any difference in soft tissue volume according to the insertion technique [
24].
A significant finding of the
in vivo study was that augmentation of the soft tissue volume was clinically observed in all animals. The augmentation area changed from an initial flattened shape to an increasingly convex shape. Notwithstanding, the analysis of the soft tissue failed to demonstrate any qualitative modification, as the soft tissue of the expanded area was of normal texture, color, and thickness; meanwhile, no increase in keratinized mucosa was achieved. Because all expanders were placed on the vestibular site of the lower jaw, no increase in keratinized tissue was to be expected, as the surrounding tissue was mainly mucosa. A previous report on the use of STEs in humans corroborated these findings and showed no alteration in the type of the original soft tissue subjected to expansion, although a significant quantitative improvement of the soft tissues was observed post-expansion [
6].
Two important histological changes occur during expansion. One is the formation of a fibrous capsule secondary to an excessive deposition of collagen matrix around implanted devices. A second event occurs at the bone surface, which serves as a counter-bearing area for the expansile stress exerted by expanders. Fibrous capsule formation is a complicated multifactorial process. The severity of capsule contracture has a positive linear correlation with the degree of local inflammatory reactions [
26]. When tissue expanders are implanted, the injury and ischemia caused by the surgical procedure initiate an acute inflammatory cascade. In the long-term expansion period, the acute inflammatory pattern becomes chronic due to the non-removable injurious stimuli from the expander [
27]. In the present study, highly vascularized, inflammation-free, collagen-rich connective tissue was clearly present at the periphery of all tissue expanders at 28 days after subperiosteal installation. While data from the early literature suggested that dense fibrous capsules could develop around the tissue expanders, completely surrounding them within a few days after insertion [
2829], more recent reports have shown that soft tissue capsules did not form unless the expander was left in its location for more than 2 weeks. This may suggest, for this study, that no fibrous encapsulation, or a lesser extent of a tissue reaction, could be found around an STE left in place for a shorter duration [
61030]. Controversy also exists regarding the impact of this reactive fibrous encapsulation on alveolar augmentation outcomes. Because the replacement of the periosteum by collagen-rich connective tissue lacking osteoblasts and precursor cells might have negative effects on the healing of the subsequent bone graft [
7], some authors have recommended leaving the expanders
in situ for only a short period of time [
30], while others have suggested inserting the STE in a submucosal pouch without elevation of the periosteum [
10]. In contrast, von See et al. [
8] showed in an
in vivo study that the reactive capsule had a significantly higher density of micro-vessels than healthy periosteum and concluded that no negative effects on vascularization to the bone were to be expected. This assumption was supported by a recent clinical trial that reported no negative outcomes following bone augmentation and implant placement, despite the development of a soft tissue capsule around the sub-periosteal expanders [
6].
It has been previously reported that STEs induced bone resorption [
612], whereas bone apposition was documented in other studies [
913]. In the present study, soft tissue expansion resulted in both outcomes. Areas of new bone formation, as well as bone resorption, could be histologically visualized in the present study, similarly to previous reports [
31]. Evidence for bone formation was found at the periphery of the expander. Similarly to our findings, Abrahamsson et al. [
913] demonstrated, in a series of experiments on rabbits, that intraoral soft tissue expansion resulted in bone apposition at the edges of the device. Because soft tissues are raised least at the margins, the slow lifting of the periosteum activates osteogenic cells that create new bone, as seen in periosteal distraction [
32]. However, in the regions of alveolar bone below the tissue expander, lacunar craters demonstrated restorative activity at the expander/bone interface. Pressure peaks appear to be among the underlying causes, as osteoclastic activity increases in areas subjected to higher pressure, especially when a certain threshold level is exceeded [
23]. Bone resorption was also attributed to the position of the expander inserted directly in contact with the bone, diminishing the vascular supply provided by the overlying periosteum. Interestingly, inserting the expanders into submucosal pouches to avoid positioning the expander between the bone and periosteum not only prevents bone resorption underneath the STE, but also results in new bone formation in humans [
10]. An additional parameter that influences bone reaction is the duration of expansion. Osteoclastic activity was demonstrated underneath STEs starting at 2 weeks postoperatively in dogs. Bone resorption then accelerated when the STE was left for more than 1 month
in situ [
12]. In light of these observations, it might be suggested that leaving the subperiosteal expanders in place for a shorter duration would counterbalance bone resorption for the STEs in the present study. Further studies are needed to thoroughly investigate the bone surface reaction to applied soft tissue expansion. Preventing the deleterious effect of bone resorption is required to optimize the outcomes of soft tissue expansion.
To provide osmotically-powered tissue expansion, we demonstrated that devices consisting of hydrogels enveloped with a N-vinyl pyrrolidone-perforated membrane provided a swelling ratio, as well as compressive and tensile strength, sufficient to induce a stable increase in the amount of oral soft tissue in dogs. Overall, after 28 days of subperiosteal insertion, fibrous encapsulation was found to replace periosteum at the periphery of the implants, while the underlying bone reaction consisted of resorption beneath the devices and apposition at their margins. More studies are required to determine the compression threshold of bone to prevent bone loss, as well as the impact of a shorter expansion period on the tissue reaction.









 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download