INTRODUCTION
MATERIALS AND METHODS
Human epithelial cell culture
Culture of epithelial cell monolayers and measurement of transepithelial electrical resistance (TER)
The cell counting kit (CCK)-8 assay
Immunofluorescence staining and confocal microscopy
Statistics
RESULTS
E2 facilitates the formation of a physical barrier in oral epithelial cell monolayers
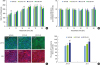 | Figure 1Facilitation of barrier formation by E2 in oral epithelial cell monolayers. (A) Immortalized human oral keratinocyte (HOK-16B) cells seeded on the membrane of a transwell 2-chamber tissue culture system were treated with various concentrations of E2 during barrier formation. The TER was measured at the indicated time points and expressed as percentage of the baseline (0 hours). (B) HOK-16B cells plated onto 96-well plates in parallel were treated and cultured in the same way. The proliferation of HOK-16B cells was measured using the CCK-8 assay kit at the indicated time points. The measured absorbances are expressed as the relative index compared to the vehicle control at each time point. (C) HOK-16B cells plated on collagen coated cover slips were treated with 2 or 20 nM E2 for 24 hours. After fixation, cells were stained for JAM-A and ZO-1 and examined by confocal microscopy with serial z-sections. Images with a maximal intensity that combined the serial z-sections are shown. (D) The fluorescence intensities of JAM-A and ZO-1 were analyzed and normalized to the fluorescence intensity of Hochest 33342.E2: estradiol, TER: transepithelial electrical resistance, CCK: cell counting kit, JAM: junctional adhesion molecule, ZO: zonula occludens, TJ: tight junction.
a)P<0.05 versus vehicle control (2-tailed non-paired Student's t-test).
|
E2 protects against TNFα-induced damage in the barrier function of oral epithelial cell monolayers
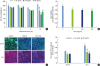 | Figure 2Disruption of the physical barrier by TNFα. Tight-junctioned monolayers of human oral keratinocyte (HOK-16B) cells were treated with various concentrations of TNFα for 24 hours. (A) The TER was measured at the indicated time points. (B) Cell viability was measured using the CCK-8 assay kit at 24 hours. (C) After fixation, cells were stained for JAM-A and ZO-1 and examined by confocal microscopy. (D) The fluorescence intensities of JAM-A and ZO-1 were analyzed.TNFα: tumor necrosis factor alpha, TER: transepithelial electrical resistance, CCK: cell counting kit, JAM: junctional adhesion molecule, ZO: zonula occludens, TJ: tight junction.
a)P<0.05 versus 0 ng/mL (2-tailed non-paired Student's t-test).
|
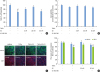 | Figure 3Protective effect of E2 on the TNFα-induced damage in oral epithelial physical barrier. Tight-junctioned monolayers of human oral keratinocyte (HOK-16B) cells were pre-treated with either ICI 182,780 for 6 hours and/or E2 for 4 hours and then treated with 100 ng/mL TNFα for 24 hours. (A) The TER was measured at the end point and expressed as the percentage of baseline. (B) Cell viability was measured using the CCK-8 assay kit at 24 hours. (C) After fixation, cells were stained for JAM-A and ZO-1 and examined by confocal microscopy. (D) The fluorescence intensities of JAM-A and ZO-1 were analyzed.E2: estradiol, TER: transepithelial electrical resistance, TNFα: tumor necrosis factor alpha, CCK: cell counting kit, JAM: junctional adhesion molecule, ZO: zonula occludens, TJ: tight junction.
a)P<0.05 versus no treatment (1-way analysis of variance with the Tukey post hoc test).
|
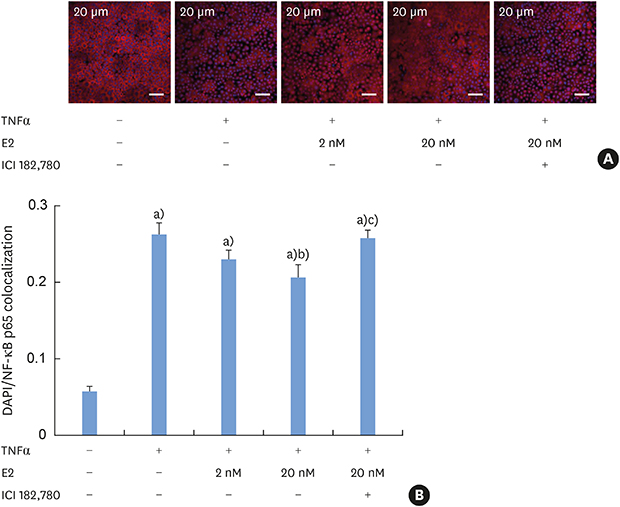
E2 inhibits the TNFα-induced nuclear translocation of NF-κB
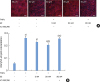 | Figure 4Inhibition of the TNFα-induced nuclear translocation of NF-κB by E2 (A) Tight-junctioned monolayers of human oral keratinocyte (HOK-16B) cells were pre-treated with either ICI 182,780 for 6 hours and/or E2 for 4 hours and then treated with 100 ng/mL TNFα for 30 minutes. After fixation, cells were stained for NF-κB p65 and examined by confocal microscopy. (B) Co-localized signals of nucleus and NF-κB p65 were measured.E2: estradiol, TNFα: tumor necrosis factor alpha.
a)P<0.0001 versus no treatment; b)P<0.05 versus treatment with TNFα alone; c)P<0.01 versus treatment with TNFα and 20 nM E2 (1-way analysis of variance with the Tukey post hoc test).
|
E2 and corticosteroids act additively on barrier formation in oral epithelial cells
 | Figure 5Additive effect of E2 and Dexa on the barrier formation in oral epithelial cell monolayers (A) Human oral keratinocyte (HOK-16B) cells seeded on the membrane of a transwell 2-chamber tissue culture system were treated with 20 nM E2, 250 nM Dexa, or E2+Dexa during barrier formation. The TER was measured at the indicated time points. (B) The proliferation of HOK-16B cells at 24 h was measured using the CCK-8 assay kit.E2: estradiol, Dexa: dexamethasone, TER: transepithelial electrical resistance, CCK: cell counting kit.
a)P<0.05 and b)P<0.0001 versus vehicle control (1-way analysis of variance with the Tukey post hoc test).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download