1. Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the Sinus Consensus Conference of 1996. Int J Oral Maxillofac Implants. 1998; 13:Suppl. 11–45.
2. Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003; 8:328–343.

3. Peleg M, Mazor Z, Garg AK. Augmentation grafting of the maxillary sinus and simultaneous implant placement in patients with 3 to 5 mm of residual alveolar bone height. Int J Oral Maxillofac Implants. 1999; 14:549–556.
4. Peleg M, Mazor Z, Chaushu G, Garg AK. Sinus floor augmentation with simultaneous implant placement in the severely atrophic maxilla. J Periodontol. 1998; 69:1397–1403.

5. Felice P, Pistilli R, Piattelli M, Soardi E, Barausse C, Esposito M. 1-stage versus 2-stage lateral sinus lift procedures: 1-year post-loading results of a multicentre randomised controlled trial. Eur J Oral Implantology. 2014; 7:65–75.
6. Zitzmann NU, Schärer P. Sinus elevation procedures in the resorbed posterior maxilla. Comparison of the crestal and lateral approaches. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:8–17.
7. Jung UW, Hong JY, Lee JS, Kim CS, Cho KS, Choi SH. A hybrid technique for sinus floor elevation in the severely resorbed posterior maxilla. J Periodontal Implant Sci. 2010; 40:76–85.

8. Felice P, Scarano A, Pistilli R, Checchi L, Piattelli M, Pellegrino G, et al. A comparison of two techniques to augment maxillary sinuses using the lateral window approach: rigid synthetic resorbable barriers versus anorganic bovine bone. Five-month post-loading clinical and histological results of a pilot randomised controlled clinical trial. Eur J Oral Implantology. 2009; 2:293–306.
9. Choi Y, Lee JS, Kim YJ, Kim MS, Choi SH, Cho KS, et al. Recombinant human bone morphogenetic protein-2 stimulates the osteogenic potential of the Schneiderian membrane: a histometric analysis in rabbits. Tissue Eng Part A. 2013; 19:1994–2004.

10. Seeherman H, Wozney J, Li R. Bone morphogenetic protein delivery systems. Spine. 2002; 27:S16–S23.

11. Jung IH, Lim HC, Lee EU, Lee JS, Jung UW, Choi SH. Comparative analysis of carrier systems for delivering bone morphogenetic proteins. J Periodontal Implant Sci. 2015; 45:136–144.

12. Alam I, Asahina I, Ohmamiuda K, Enomoto S. Comparative study of biphasic calcium phosphate ceramics impregnated with rhBMP-2 as bone substitutes. J Biomed Mater Res. 2001; 54:129–138.

13. Brodie JC, Goldie E, Connel G, Merry J, Grant MH. Osteoblast interactions with calcium phosphate ceramics modified by coating with type I collagen. J Biomed Mater Res A. 2005; 73:409–421.

14. Kim JS, Cha JK, Cho AR, Kim MS, Lee JS, Hong JY, et al. Acceleration of bone regeneration by BMP-2-loaded collagenated biphasic calcium phosphate in rabbit sinus. Clin Implant Dent Relat Res. 2015; 17:1103–1113.

15. Watanabe K, Niimi A, Ueda M. Autogenous bone grafts in the rabbit maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88:26–32.

16. Wada K, Niimi A, Watanabe K, Sawai T, Ueda M. Maxillary sinus floor augmentation in rabbits: a comparative histologic-histomorphometric study between rhBMP-2 and autogenous bone. Int J Periodontics Restorative Dent. 2001; 21:252–263.
17. Sun XJ, Zhang ZY, Wang SY, Gittens SA, Jiang XQ, Chou LL. Maxillary sinus floor elevation using a tissue-engineered bone complex with OsteoBone and bMSCs in rabbits. Clin Oral Implants Res. 2008; 19:804–813.

18. Kim YS, Kim SH, Kim KH, Jhin MJ, Kim WK, Lee YK, et al. Rabbit maxillary sinus augmentation model with simultaneous implant placement: differential responses to the graft materials. J Periodontal Implant Sci. 2012; 42:204–211.

19. Brunner E, Langer F. Nonparametric analysis of ordered categorical data in designs with longitudinal observations and small sample sizes. Biom J. 2000; 42:663–675.

20. Wikesjö UM, Qahash M, Thomson RC, Cook AD, Rohrer MD, Wozney JM, et al. rhBMP-2 significantly enhances guided bone regeneration. Clin Oral Implants Res. 2004; 15:194–204.

21. Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992; 74:659–670.

22. Sigurdsson TJ, Fu E, Tatakis DN, Rohrer MD, Wikesjö UM. Bone morphogenetic protein-2 for peri-implant bone regeneration and osseointegration. Clin Oral Implants Res. 1997; 8:367–374.

23. Guillot R, Gilde F, Becquart P, Sailhan F, Lapeyrere A, Logeart-Avramoglou D, et al. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials. 2013; 34:5737–5746.

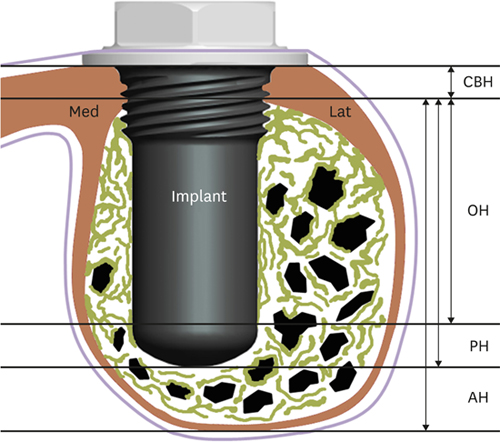
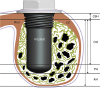
24. Jung UW, Unursaikhan O, Park JY, Lee JS, Otgonbold J, Choi SH. Tenting effect of the elevated sinus membrane over an implant with adjunctive use of a hydroxyapatite-powdered collagen membrane in rabbits. Clin Oral Implants Res. 2015; 26:663–670.

25. Chen D, Zhao M, Harris SE, Mi Z. Signal transduction and biological functions of bone morphogenetic proteins. Front Biosci. 2004; 9:349–358.

26. Khoury F. Augmentation of the sinus floor with mandibular bone block and simultaneous implantation: a 6-year clinical investigation. Int J Oral Maxillofac Implants. 1999; 14:557–564.










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download