INTRODUCTION
MATERIALS AND METHODS
Preparation of rough substrates
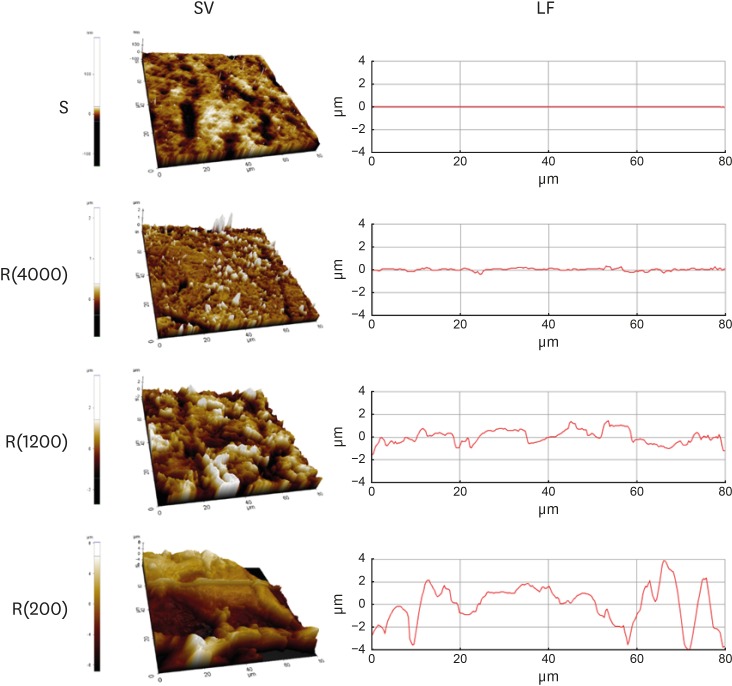 | Figure 1Model substrates. Substrates prepared in polystyrene dishes with varying levels of roughness were analyzed using atomic force microscopy. SV and LF of the substrates with varying levels of roughness.
SV: surface views, LF: line profiles, S: smooth culture dish, R(4000): prepared with #4000 sandpaper, R(1200): prepared with #1200 sandpaper, R(200): prepared with #200 sandpaper.
|
Table 1
Roughnesses of the susbtrates for cultures of HOK-16B cells
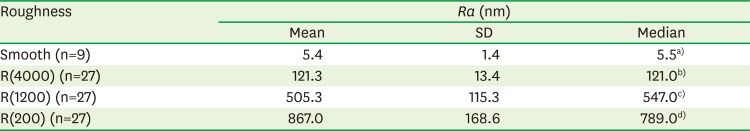
| Roughness | Ra (nm) | ||
|---|---|---|---|
| Mean | SD | Median | |
| Smooth (n=9) | 5.4 | 1.4 | 5.5a) |
| R(4000) (n=27) | 121.3 | 13.4 | 121.0b) |
| R(1200) (n=27) | 505.3 | 115.3 | 547.0c) |
| R(200) (n=27) | 867.0 | 168.6 | 789.0d) |
Reagents
Cell cultures and transfections
Field emission scanning electron microscopic observation
Immunoblotting
Immunocytochemistry
Statistical analysis
RESULTS
Limited and delayed spreading of oral keratinocytes on rough substrates
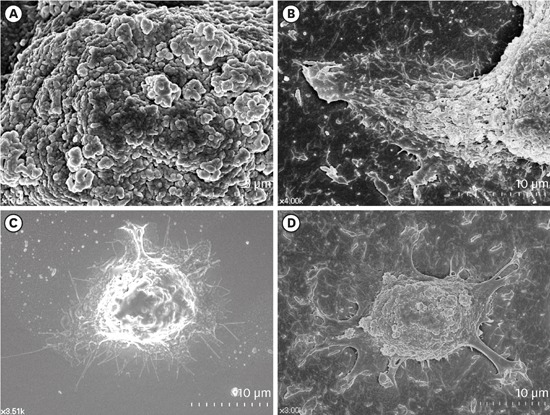
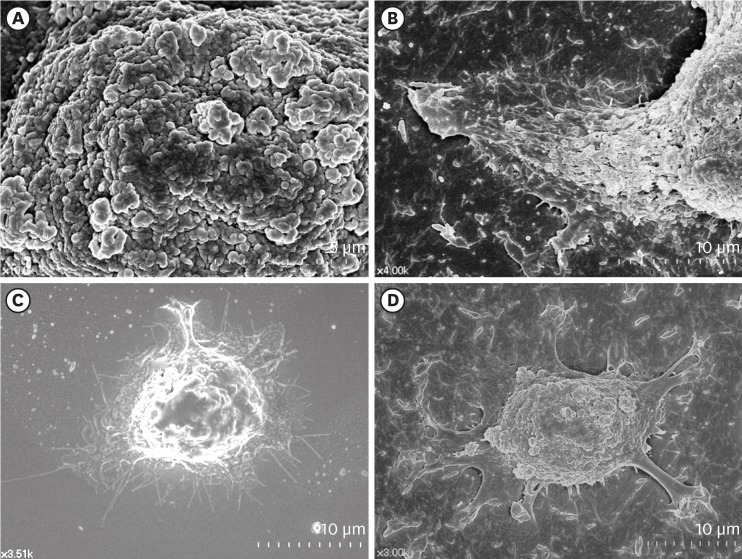 | Figure 2Surface structures of HGKs attached to smooth substrates or substrates of low-nanometer dimensions (Ra=121.3±13.4 nm). Cells were processed for observation by FE-SEM 10 hours after cell seeding. (A) Membrane folding and furrows are rich on the surface of the underside of the cell bodies on the rough substrate (bar=5 µm). (B) Membrane folding and furrows gradually fade toward the end of the cell process, which is extended from the cell body on the rough substrate (bar=10 µm). (C) The cells on the smooth substrate display circumferential lamellipodia rich in filopodia (bar=10 µm). (D) Several cell processes extend from the cell body on the rough substrate. Except for the end of the cell processes, the margin of the cell membrane is linear without filopodia. The surface of the cell body is rich in membrane folding and furrows (bar=10 µm).
HGK: human gingival keratinocyte, Ra: average roughness, FE-SEM: field emission scanning electron microscopy.
|
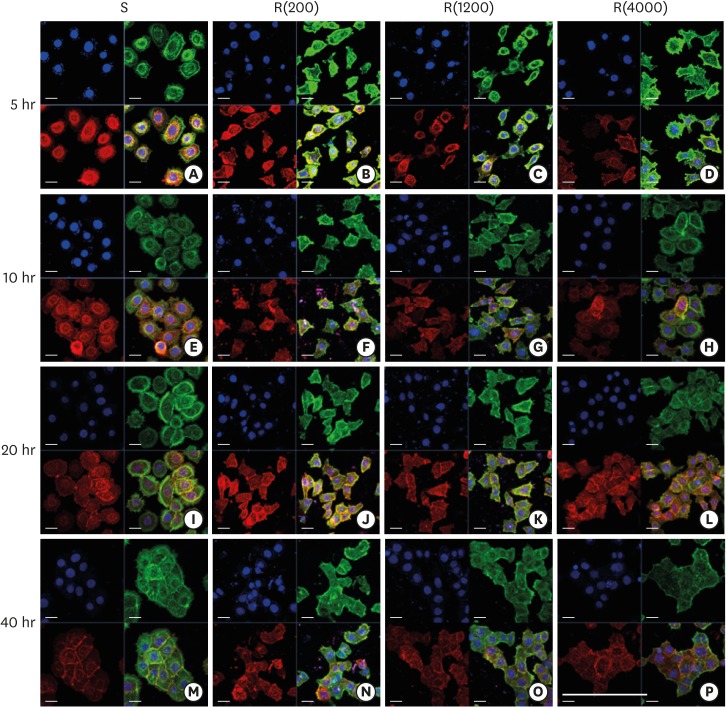 | Figure 3Development of the ECJs of HGKs depends on the substrate roughness in vitro, as shown by confocal laser-scanning microscopy. ECJ development was followed by immunocytochemical staining for the expression level of E-cadherin (red) at 5, 10, 20, and 40 hours after cell seeding. F-actin (green) was stained with FITC-phalloidin. Nuclei were stained with DAPI (blue). The lower right picture in each set of 4 pictures is a merged image of the E-cadherin, F-actin, and nuclei images. Refer to the results section for details regarding ECJ development (short bar=20 µm, long bar=160 µm).
ECJ: E-cadherin junction, HGK: human gingival keratinocyte, FITC: fluorescein isothiocyanate-labeled, DAPI: 4′, 6-diamidino-2-phenylindole dihydrochloride, S: smooth culture dish, R(4000): prepared with #4000 sandpaper, R(1200): prepared with #1200 sandpaper, R(200): prepared with #200 sandpaper.
|
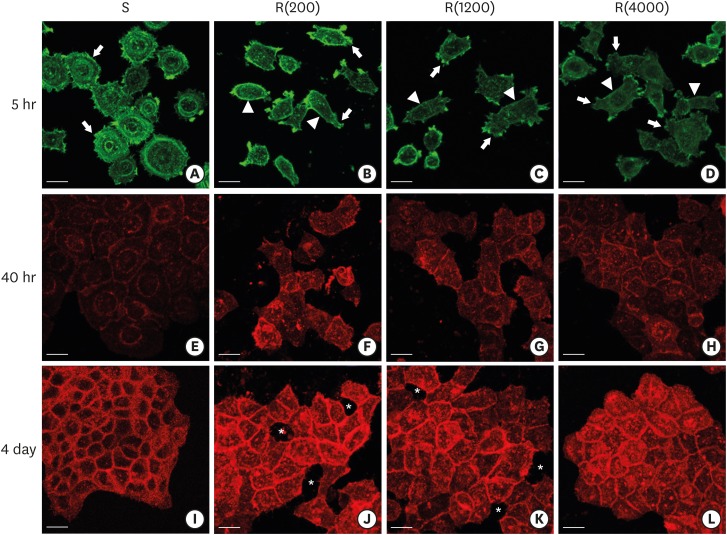 | Figure 4Development of the ECJs of HGKs depended on the substrate roughness in vitro, as shown by confocal laser-scanning microscopy. (A-D) F-actin (green) in HOK-16B cells cultured on the substrates with varying levels of roughness for 5 hours was stained with FITC-phalloidin to identify cortical actin (arrow heads) or lamellipodia (arrows). (E-H) E-cadherin (red) in HOK-16B cells cultured on substrates with varying levels of roughness for 40 hours was immunohistochemically stained to examine how ECJ development depended on the roughness of the substrates. (I-L) E-cadherin (red) of HOK-16B cells cultured on substrates with varying levels of roughness for 4 days were immunohistochemically stained to examine how ECJ development depended on the roughness of the substrates. Intercellular gaps (*) are clearly present between cells in (J) and (K). Refer to the results section for details regarding ECJ development (short bar=20 µm). Ra=121.3±13.4, 505.3±115.3, and 867.0±168.6 nm for R(4000), R(1200), and R(200), respectively.
ECJ: E-cadherin junction, HGK: human gingival keratinocyte, FITC: fluorescein isothiocyanate-labeled, Ra: average roughness, R(4000): prepared with #4000 sandpaper, R(1200): prepared with #1200 sandpaper, R(200): prepared with #200 sandpaper, S: smooth substrate.
|
Defective intercellular junction development in HGKs cultured on rough substrates
Table 2
Development of ECJs of HOK-16B cells depending on the substrate roughness in vitro
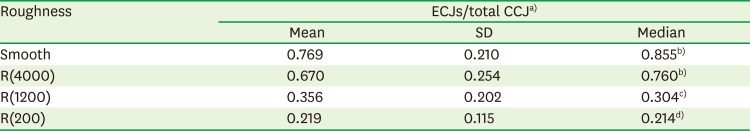
| Roughness | ECJs/total CCJa) | ||
|---|---|---|---|
| Mean | SD | Median | |
| Smooth | 0.769 | 0.210 | 0.855b) |
| R(4000) | 0.670 | 0.254 | 0.760b) |
| R(1200) | 0.356 | 0.202 | 0.304c) |
| R(200) | 0.219 | 0.115 | 0.214d) |
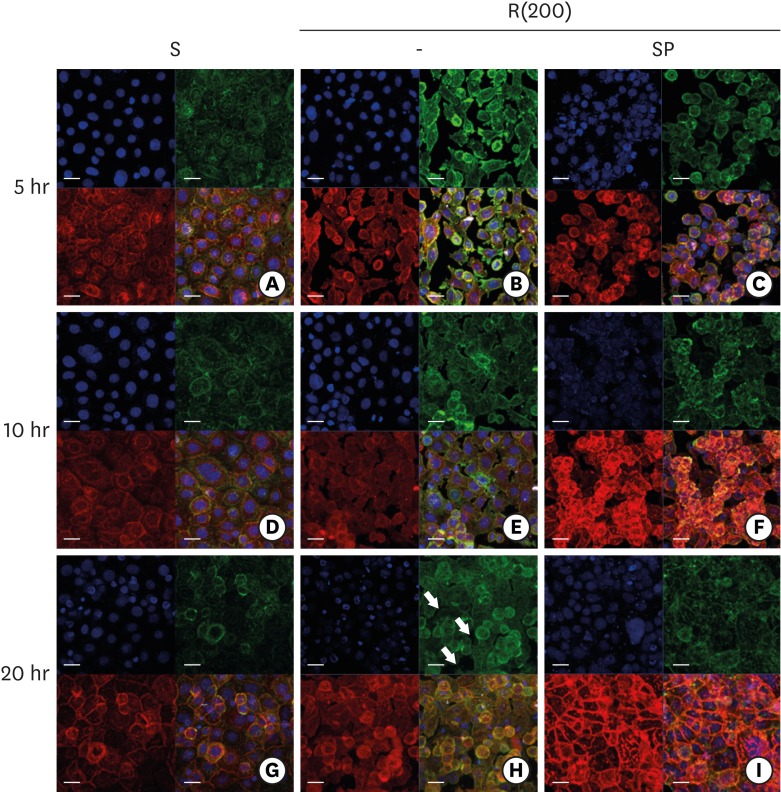 | Figure 5ECJ development in HGKs seeded at a high density on smooth or rough substrates was examined using confocal laser-scanning microscopy. ECJ development was followed by immunocytochemical staining for the expression level of E-cadherin (red) at 5, 10, and 20 hours after cell seeding. F-actin (green) was stained with FITC-phalloidin. Nuclei were stained with DAPI (blue). The lower right picture in each set of 4 pictures is a merged image of the E-cadherin, F-actin, and nuclei images. (A, D, G) Cells on the smooth substrates were cultured under normal conditions. Cells on the rough substrates with high-nanometer dimensions, R(200), were cultured under normal conditions (B, E, H) or JNK inhibition by 1 µM SP (C, F, I). (H) Arrows indicate the wide intercellular gaps remaining between the cells on the rough substrate with a high-nanometer dimension despite the high density of the culture (bar=20 µm). Ra=867.0±168.6 nm for R(200).
ECJ: E-cadherin junction, HGK: human gingival keratinocyte, FITC: fluorescein isothiocyanate-labeled, DAPI: 4′, 6-diamidino-2-phenylindole dihydrochloride, JNK: c-Jun N-terminal kinase, S: smooth substrate, SP: SP600125, Ra: average roughness, R(200): prepared with #200 sandpaper.
|
Low stability of ECJs in cells cultured on rough substrates
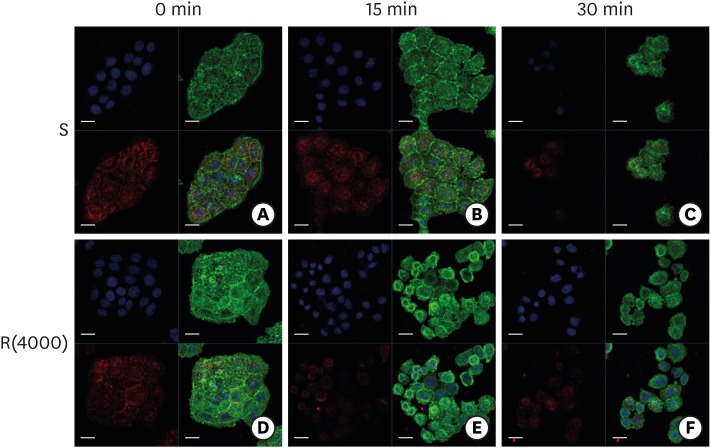 | Figure 6Dissociation of ECJs after calcium depletion. HGKs were treated for the indicated times with 0.25% trypsin-EDTA after culturing for 4 days on the smooth substrate (A-C) or the rough substrate with low-nanometer dimensions (D-F). ECJ development was followed by immunocytochemical staining for the expression level of E-cadherin (red) at 0, 15, and 30 minutes after calcium depletion. F-actin (green) was stained with FITC-phalloidin. Nuclei were stained with DAPI (blue). The lower right picture in each set of 4 pictures is a merged image of the E-cadherin, F-actin, and nuclei images (bar=20 µm).
ECJ: E-cadherin junction, HGK: human gingival keratinocyte, FITC: fluorescein isothiocyanate-labeled, DAPI: 4′, 6-diamidino-2-phenylindole dihydrochloride, S: smooth substrate, R(4000): prepared with #4000 sandpaper.
|
Role of JNK in ECJ development in HGKs cultured on rough surfaces
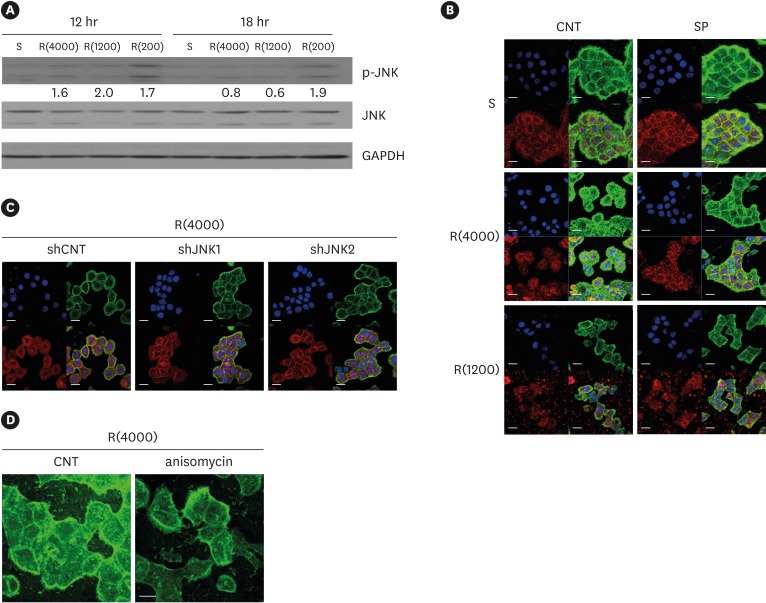 | Figure 7Influence of JNK on ECJ development. (A) HGKs were cultured on substrates with varying levels of roughness. Then, the expression levels of p-JNK were compared by western blotting. Values under the blots for p-JNK indicate the relative expression level of p-JNK to the total JNK for each group, which was analyzed using Image J. (B) HGKs were treated with SP (1 µM) to inhibit JNK activity for 24 hours in culture starting 12 hours after cell seeding on the smooth and rough substrates. CNT indicates the control culture without treatments with SP. (C) HGKs transfected using lentiviruses expressing shJNK1/2 to selectively inhibit JNK1/2 activity were re-plated for 24 hours on the rough substrate with low-nanometer dimensions (Ra=121.3±13.4 nm). ECJ development was followed by immunocytochemical staining for the expression level of E-cadherin (red). F-actin (green) was stained with FITC-phalloidin. Nuclei were stained with DAPI (blue). The lower right picture in each set of 4 pictures is a merged image of the E-cadherin, F-actin, and nuclei images. shCNT, scrambled shRNA. (D) HGKs were treated with anisomycin (20 ng/mL) to activate JNK for 20 hours in culture starting 3 days after cell seeding on the rough substrates. CNT indicates the control culture without treatments with anisomycin (bar=20 µm). Ra=121.3±13.4, 505.3±115.3, and 867.0±168.6 nm for R(4000), R(1200), and R(200), respectively.
S: smooth substrate, SP: SP600125, JNK: c-Jun N-terminal kinase, ECJ: E-cadherin junction, HGK: human gingival keratinocyte, CNT: carbon nanotube, Ra: average roughness, p-JNK: phospho-c-Jun N-terminal kinase, shRNA: small hairpin RNA, FITC: fluorescein isothiocyanate-labeled, DAPI: 4′, 6-diamidino-2-phenylindole dihydrochloride, R(4000): prepared with #4000 sandpaper, R(1200): prepared with #1200 sandpaper, R(200): prepared with #200 sandpaper.
|
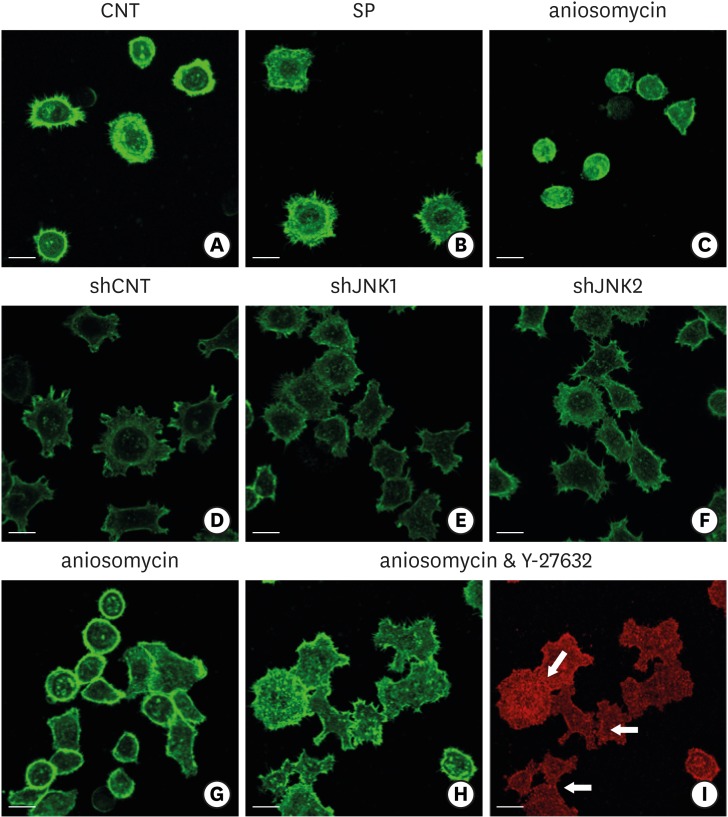 | Figure 8Influence of JNK on the organization of F-actin during ECJ development. (A-C) The development of cortical actin was assessed using CLSM after treating the cells with SP (1 µM) (B), or anisomycin (20 ng/mL) (C) for 20 hours from the start of cell culture on the smooth substrate. CNT (A) indicates the control culture without treatments regulating JNK activity. F-actin was stained with FITC-phalloidin (bar=20 µm). (D-F) Cortical actin development was assessed using CLSM after HGKs infected with lentiviruses expressing shJNK1 (E) or shJNK2 (F) to selectively inhibit JNK1 or JNK2 were cultured for 8 hours on the rough substrate with low-nanometer dimensions (Ra=121.3±13.4 nm). Control cells (shCNT) (D) were cultured after transfection with scrambled shRNA. F-actin was stained with FITC-phalloidin (bar=20 µm). (G-I) Cortical actin development and ECJ development (arrows) were assessed using CLSM after treating the cells with anisomycin (20 ng/mL) (G) or co-treating the cells with anisomycin (20 ng/mL) and Y-27632 (20 µM) (H, I) for 20 hours from the start of cell culture on the smooth substrate. F-actin (green) was stained with FITC-phalloidin. ECJ development was followed by immunocytochemical staining for the expression level of E-cadherin (red) (bar=20 µm).
JNK: c-Jun N-terminal kinase, ECJ: E-cadherin junction, CNT: carbon nanotube, Ra: average roughness, SP: SP600125, CLSM: confocal laser-scanning microscopy, FITC: fluorescein isothiocyanate-labeled, sh: small hairpin.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download