1. De Bruyn H, Vandeweghe S, Ruyffelaert C, Cosyn J, Sennerby L. Radiographic evaluation of modern oral implants with emphasis on crestal bone level and relevance to peri-implant health. Periodontol 2000. 2013; 62:256–270. PMID:
23574471.

2. Christiaens V, De Bruyn H, Thevissen E, Koole S, Dierens M, Cosyn J. Assessment of periodontal bone level revisited: a controlled study on the diagnostic accuracy of clinical evaluation methods and intra-oral radiography. Clin Oral Investig. 2018; 22:425–431.

3. Christiaens V, Jacobs R, Dierens M, Vervaeke S, De Bruyn H, Koole S, et al. Intraoral radiography lacks accuracy for the assessment of peri-implant bone level - a controlled clinical study. Eur J Oral Implantology. 2017; 10:435–441.
4. García-García M, Mir-Mari J, Benic GI, Figueiredo R, Valmaseda-Castellón E. Accuracy of periapical radiography in assessing bone level in implants affected by peri-implantitis: a cross-sectional study. J Clin Periodontol. 2016; 43:85–91. PMID:
26660842.

5. Bohner LO, Mukai E, Oderich E, Porporatti AL, Pacheco-Pereira C, Tortamano P, et al. Comparative analysis of imaging techniques for diagnostic accuracy of peri-implant bone defects: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017; 124:432–440.e5. PMID:
28743664.

6. Agrawal P, Sanikop S, Patil S. New developments in tools for periodontal diagnosis. Int Dent J. 2012; 62:57–64. PMID:
22420472.

7. Mota CC, Fernandes LO, Cimões R, Gomes AS. Non-invasive periodontal probing through fourier-domain optical coherence tomography. J Periodontol. 2015; 86:1087–1094. PMID:
25879790.

8. Colston BW Jr, Everett MJ, Da Silva LB, Otis LL, Stroeve P, Nathel H. Imaging of hard- and soft-tissue structure in the oral cavity by optical coherence tomography. Appl Opt. 1998; 37:3582–3585. PMID:
18273327.

9. Fernandes LO, Mota CC, de Melo LS, da Costa Soares MU, da Silva Feitosa D, Gomes AS.
In vivo assessment of periodontal structures and measurement of gingival sulcus with Optical Coherence Tomography: a pilot study. J Biophotonics. 2017; 10:862–869. PMID:
27503608.
10. Hsieh YS, Ho YC, Lee SY, Lu CW, Jiang CP, Chuang CC, et al. Subgingival calculus imaging based on swept-source optical coherence tomography. J Biomed Opt. 2011; 16:071409. PMID:
21806255.

11. Park JY, Chung JH, Lee JS, Kim HJ, Choi SH, Jung UW. Comparisons of the diagnostic accuracies of optical coherence tomography, micro-computed tomography, and histology in periodontal disease: an
ex vivo study. J Periodontal Implant Sci. 2017; 47:30–40. PMID:
28261522.
12. Kim SH, Kang SR, Park HJ, Kim JM, Yi WJ, Kim TI. Improved accuracy in periodontal pocket depth measurement using optical coherence tomography. J Periodontal Implant Sci. 2017; 47:13–19. PMID:
28261520.

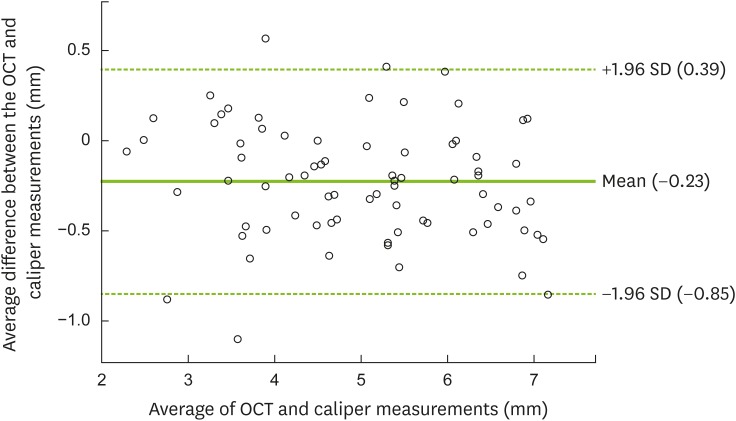
13. Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician. 1983; 32:307–317.

14. Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981; 10:387–416. PMID:
6809663.

15. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986; 1:11–25. PMID:
3527955.
16. Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992; 3:9–16. PMID:
1420727.

17. Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 2010; 53:167–181. PMID:
20403112.

18. Serino G, Turri A, Lang NP. Probing at implants with peri-implantitis and its relation to clinical peri-implant bone loss. Clin Oral Implants Res. 2013; 24:91–95. PMID:
22462625.

19. Ringeling J, Parvini P, Weinbach C, Nentwig GH, Nickles K, Eickholz P. Discomfort/pain due to pocket probing at teeth and endosseous implants: a cross-sectional study. Clin Oral Implants Res. 2016; 27:1005–1009. PMID:
26183155.

20. Benn DK. A review of the reliability of radiographic measurements in estimating alveolar bone changes. J Clin Periodontol. 1990; 17:14–21. PMID:
2404031.

21. Coli P, Christiaens V, Sennerby L, Bruyn H. Reliability of periodontal diagnostic tools for monitoring peri-implant health and disease. Periodontol 2000. 2017; 73:203–217. PMID:
28000267.

22. Tomasi C, Derks J. Clinical research of peri-implant diseases--quality of reporting, case definitions and methods to study incidence, prevalence and risk factors of peri-implant diseases. J Clin Periodontol. 2012; 39(Suppl 12):207–223. PMID:
22533958.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download