1. Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012; 67(1):18–24.
2. Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008; 100(3):Suppl 3. S1–S148.

3. Adkinson NF, Middleton E. Middleton's Allergy: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier/Saunders;2014.
4. Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008; 38(8):1400–1408.

5. Nielsen NH, Dirksen A, Mosbech H, Launbjerg J, Biering I, Soborg M. Skin prick testing with standardized extracts from 3 different manufacturers. A comparative randomized study. Allergol Immunopathol (Madr). 1992; 20(6):246–248.
6. Meyer CH, Bond JF, Chen MS, Kasaian MT. Comparison of the levels of the major allergens Der-P-I and Der-P-Ii in standardized extracts of the house-dust mite, Dermatophagoides-pteronyssinus. Clin Exp Allergy. 1994; 24(11):1041–1048.
7. Larsen JN, Houghton CG, Vega ML, Lowenstein H. Manufacturing and standardizing allergen extracts in Europe. Clin Allergy Immunol. 2008; 21:283–301.
8. Jeong KY, Son M, Park JH, Park KH, Park HJ, Lee JH, et al. Cross-reactivity between oak and birch pollens in Korean tree pollinosis. J Korean Med Sci. 2016; 31(8):1202–1207.

9. Jeong KY, Park JW, Hong CS. House dust mite allergy in Korea: the most important inhalant allergen in current and future. Allergy Asthma Immunol Res. 2012; 4(6):313–325.

10. Kim TB, Kim KM, Kim SH, Kang HR, Chang YS, Kim CW, et al. Sensitization rates for inhalant allergens in Korea; a multi-center study. J Asthma Allergy Clin Immunol. 2003; 23(3):483–493.
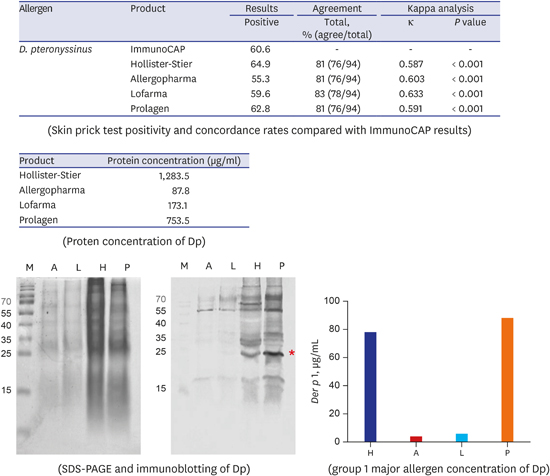
11. Jeong KY, Choi SY, Lee JH, Lee IY, Yong TS, Lee JS, et al. Standardization of house dust mite extracts in Korea. Allergy Asthma Immunol Res. 2012; 4(6):346–350.

12. Jeong KY, Son M, Choi SY, Park KH, Park HJ, Hong CS, et al. Standardization of weed pollen extracts, Japanese hop and mugwort, in Korea. Yonsei Med J. 2016; 57(2):399–406.

13. Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011; 52(3):393–400.

14. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33(1):159–174.

15. Eichler I, Gotz M, Jarisch R, Eichler HG, Moss R. Reproducibility of skin prick testing with allergen extracts from different manufacturers. Allergy. 1988; 43(6):458–463.

16. Oh JW. Characteristics of allergic pollens and the recent increase of sensitization rate to weed pollen in childhood in Korea. Korean J Pediatr. 2008; 51(4):355–361.

17. Liu ZG, Song JJ, Kong XL. A study on pollen allergens in China. Biomed Environ Sci. 2010; 23(4):319–322.

18. Park HJ, Lee JH, Park KH, Kim KR, Han MJ, Choe H, et al. A six-Year study on the changes in airborne pollen counts and skin positivity rates in Korea: 2008–2013. Yonsei Med J. 2016; 57(3):714–720.

19. Jin HJ, Choi GS, Shin YS, Kim JH, Kim JE, Ye YM, et al. The Allergenic Potency of Japanese hop pollen is increasing with environmental changes in Korea. Allergy Asthma Immunol Res. 2013; 5(5):309–314.

20. Bjerg A, Winberg A, Berthold M, Mattsson L, Borres MP, Ronmark E. A population-based study of animal component sensitization, asthma, and rhinitis in schoolchildren. Pediatr Allergy Immunol. 2015; 26(6):557–563.

21. Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011; 154(3):258–263.

22. Nam YH, Lee SK. Comparison between skin prick test and serum immunoglobulin E by CAP system to inhalant allergens. Ann Allergy Asthma Immunol. 2017; 118(5):608–613.

23. Park HJ, Lee JH, Park KH, Ann HW, Jin MN, Choi SY, et al. A nationwide survey of inhalant allergens sensitization and levels of indoor major allergens in Korea. Allergy Asthma Immunol Res. 2014; 6(3):222–227.












 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download