INTRODUCTION
Influenza is a highly contagious acute respiratory disease caused by influenza A or B viruses. Influenza is usually accompanied by symptoms such as fever, headache, myalgia, fatigue, cough and sore throat, and usually can be recovered within 7 days. However, high-risk people like the elderly over 65 years old, pregnant women, or patients with immunocompromised diseases can have complications such as pneumonia, and can lead to death in severe cases. In the United States, more than 200,000 people are hospitalized each year for influenza.
1 In Korea, more than 23,000 people are estimated to be admitted and more than 1,200 people are estimated to die due to the influenza each year.
2 Influenza viruses have antigenic variations that cause not only the annual seasonal epidemic, but also pandemic every 10 to 40 years. Therefore, the antiviral agents against influenza are very important for the response to influenza pandemics as well as treatment of seasonal influenza.
Antiviral agents used for the treatment of influenza include amantadine and rimantadine classified as M2-blocker, and oseltamivir, zanamivir and peramivir classified as neuraminidase inhibitor. The M2-blocker is effective for influenza A but not for B, and it is more likely to have AEs relatively. In addition, since the current influenza A virus is resistant to M2-blocker, the use of M2-blocker is no longer recommended in the influenza treatment guidelines.
13 On the other hand, influenza A and B viruses circulating currently are susceptible to neuraminidase inhibitors. Therefore, the neuraminidase inhibitor is the mainstay in the treatment of influenza now.
Peramivir is one of the neuraminidase inhibitors, and is the only intravenous formulation currently available. Peramivir has the advantage that it can be administered to patients who have difficulty in taking oral (oseltamivir) or inhaled (zanamivir) agent. The single administration is another advantage in that there is no concern about low compliance, which can decrease the risk of treatment failure or development of antiviral resistance. Peramivir was approved in Japan and Korea in 2010, and the United States in 2014.
4 Clinical trials have shown that the effectiveness of peramivir was comparable to oseltamivir and the occurrence of AEs was relatively low.
56 However, the safety and clinical efficacy data of peramivir in the real clinical field is still limited. To address this, we performed a prospective observational study to evaluate the post-marketing safety and efficacy of peramivir in adults with seasonal influenza. This study was conducted in accordance with the regulation of Korea Ministry of Food and Drug Safety (KMFDS, notice No. 2013-251).
METHODS
Patients
This study was a single-arm, prospective, observational, cohort study conducted at 75 medical institutions in the Republic of Korea during December 2010 to August 2016. To be enrolled in the study, patients had to meet all the following inclusion criteria: adults aged 20 years or older at enrollment, laboratory confirmation of influenza A or B, administration of peramivir within 48 hours from the initial symptoms of influenza, and obtaining a subject's written informed consent. The laboratory confirmation of influenza was performed at each medical institution with rapid antigen test (RAT), reverse transcription polymerase chain reaction (RT-PCR) or culture. Subjects with the use of peramivir out of the formal dosage recommended by KMFDS were excluded. The formal use of peramivir in Korea is to administer 300 mg once to adult influenza patients with normal renal function. The peramivir dose should be reduced for patients with impaired renal function according to their creatinine clearance. The administration of peramivir to pregnant women is generally limited due to insufficient safety data. The decision on whether to administer peramivir was based on the clinical indication by physicians of each medical institution.
Safety assessment
All AEs were checked using the prepared check sheet, which included events that occurred within 7 days after administration of peramivir. The grade and causal relationship of AEs was assessed according to the guideline of the KMFDS (notice No. 2014-97). The AEs were graded as 1 to 4 according to the severity. Severe adverse events (SAEs) were defined as important medical events that resulted in life-threatening, death, hospitalization/prolonged hospitalization, persistent or significant disability/incapacity, congenital anomaly, or other medically important situation. The causal relationship of AEs with peramivir was classified as certain, probably/likely, possible, unlikely, conditional/unclassified, or unassessable/unclassifiable. The causality was determined by physicians of each institution. Adverse drug reactions (ADRs) were defined as AEs for which the causality could not be ruled out as determined by physicians or sponsor.
Effectiveness assessment
The condition of the enrolled patients was checked before and 7 days after the administration of peramivir. The severity of influenza symptoms was evaluated using 0–3 scale as follows: 0, normal; 1, mild (barely noticeable); 2, moderate (bothersome); 3, severe (unbearable). The influenza symptom checked included cough, sore throat, headache, nasal congestion, febrile sense or chills, muscle or joint pain, and fatigue. Daily living performance was checked using 0–10 visual analogue scale (VAS). The date on which influenza symptoms disappeared was checked. The final effectiveness of peramivir was determined by physicians of each institution, based on the symptom and sign, as follows: effective, not effective, unable to judge.
Statistical analysis
This study was a single-arm study, so most values were described without comparisons. For the sub-analysis for estimating the difference of the safety or effectiveness according to the characteristics, the χ2 test or the Fisher's exact test was used for categorical variables, and the t-test was used for continuous variable. Logistic regression analysis was performed to confirm the independent factors related to the AE or ADR occurrence. The factors with the statistical significance related to the AE or ADR occurrence in the univariate analysis were included in the logistic regression analysis. Comparing pre- and post-status of the influenza symptom severity and daily living performance for the effectiveness assessment was done by using Wilcoxon rank-sum test. A two-sided significance level of 5% was used throughout. The continuous variables contained the minimum and maximum statistics, the arithmetic mean, standard deviation and median, and the 95% confidence interval (CI). The categorical variables contained counts and percentages.
Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of the 28 medical institutions participating this study (IRB No. of Korea University Guro Hospital: KUGH14296-001). For institutions that do not have an individual IRB, they conducted this study based on the IRB approval information from other institutions. Informed consent was obtained from all subjects when they were enrolled.
RESULTS
Characteristics of the study population
A total of 4,056 check sheets were collected during the study period. Of these, 1,032 were excluded from the analysis with the reasons as follows: 974, dosage out of the formal recommendation of KMFDS; 17, duplicated cases; 65, follow-up loss; the exclusion reasons were duplicated. As a result, a total of 3,024 subjects were included in the safety assessment. For the effectiveness assessment, a total of 2,939 subjects were included because effectiveness data was not collected from 85 subjects. The number of enrolled subjects according to the influenza seasons is shown in
Fig. 1.
 | Fig. 1
The number of enrolled patients according to the influenza seasons.
aExclusion reasons were duplicated.

|
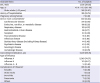
Of the 3,024 subjects, 39.9% (n = 1,205) were male (
Table 1). Mean age was 45.55 ± 15.30 years and 12.07% (n = 365) were adults aged 65 years or older. About one of ten subjects (10.75%, n = 325) had a history of hospitalization. Underlying diseases were present in 18.82% (n = 569) patients, and 69.74% (n = 2,109) had concomitant medication. The most common concomitant medications were anti-tussive (46.36%, n = 1,402), analgesics/antipyretics (41.57%, n = 1,257) and non-steroidal anti-inflammatory agents (38.36%, n = 1,160).
Table 1
Characteristics of the enrolled patients
|
Characteristics |
No. of subjects |
|
Sex, male |
1,205 (39.85) |
|
Age, yr |
45.6 ± 15.3 (20–98) |
|
Older adults (≥ 65 years) |
365 (12.07) |
|
History of hospitalization |
325 (10.75) |
|
Pregnancy among female |
7 (0.38) |
|
One or more comorbiditiesa
|
569 (18.82) |
|
Cardiovascular disease |
272 (8.99) |
|
Endocrine, nutrition, or metabolic disease |
188 (6.22) |
|
Respiratory disease |
123 (4.07) |
|
Gastrointestinal or liver disease |
96 (3.17) |
|
Neoplasm |
90 (2.98) |
|
Musculoskeletal disease |
55 (1.82) |
|
Infectious disease |
55 (1.82) |
|
Genitourinary disease (including kidney disease) |
44 (1.46) |
|
Psychiatric disease |
40 (1.32) |
|
Neurologic disease |
34 (1.12) |
|
Hematologic disease |
23 (0.76) |
|
Concomitant medication, yes |
2,109 (69.74) |
|
Type of influenza |
|
|
Influenza A |
2,249 (74.37) |
|
Influenza B |
761 (25.17) |
|
Influenza A + B |
14 (0.46) |
|
Complication of influenzaa
|
285 (9.42) |
|
Sinusitis |
11 (0.36) |
|
Otitis media |
1 (0.03) |
|
Bronchitis |
189 (6.25) |
|
Pneumonia |
71 (2.35) |
|
Others |
23 (0.76) |

Influenza A and B accounted for 74.37% (n = 2,249) and 25.17% (n = 761), respectively. Fourteen subjects (0.46%) were diagnosed with both A and B infection. Complication of influenza was present in 9.42% (n = 285) patients, and bronchitis (6.25%, n = 189) and pneumonia (2.35%, n = 71) were most common.
Safety assessment
A total of 42 AEs was observed in 1.16% (n = 35) patients (
Table 2). The most common AE was fever (0.17%, n = 5), diarrhea (0.13%, n = 4), nausea (0.10%, n = 3) and pneumonia (0.10%, n = 3). AEs were mostly rated as mild in severity (76.19%, 32/42). SAEs were observed in 10 subjects (0.33%) and two of them died. However, both deaths were attributed to pneumonia, which was less relevant to peramivir. Among the 42 AEs, only eight (19.05%) were estimated as possible causality and most (73.81%, 31/42) were assessed as unlikely. Eleven ADRs were identified in 10 patients: nausea (0.10%, n = 3), leukopenia (0.07%, n = 2), and liver function abnormality (0.07%, n = 2) were the most common ADRs.
Table 2
Incidence of AEs and ADR of peramivir

|
Category of AEs |
AEs |
ADRs |
|
No. of subjects (%), [No. of events] |
No. of subjects (%), [No. of events] |
|
Gastrointestinal disorder |
12 (0.40), [13] |
5 (0.17), [5] |
|
Diarrhea |
4 (0.13), [4] |
1 (0.03), [1] |
|
Nausea |
3 (0.10), [3] |
3 (0.10), [3] |
|
Constipation |
2 (0.07), [2] |
- |
|
Stomatitis |
1 (0.03), [1] |
- |
|
Abdominal pain |
1 (0.03), [1] |
- |
|
Upper abdominal pain |
1 (0.03), [1] |
1 (0.03), [1] |
|
Indigestion |
1 (0.03), [1] |
- |
|
Infection |
5 (0.17), [5] |
1 (0.03), [1] |
|
Pneumonia |
3 (0.10), [3] |
1 (0.03), [1] |
|
Herpes simplex |
1 (0.03), [1] |
- |
|
Skin and soft tissue infection |
1 (0.03), [1] |
- |
|
Administration reaction |
5 (0.17), [5] |
- |
|
Fever |
5 (0.17), [5] |
- |
|
Respiratory disorder |
4 (0.13), [4] |
- |
|
Rhinorrhea |
2 (0.07), [2] |
- |
|
Hemoptysis |
1 (0.03), [1] |
- |
|
Pulmonary embolism |
1 (0.03), [1] |
- |
|
Hematologic disorder |
3 (0.10), [3] |
2 (0.07), [2] |
|
Leukopenia |
2 (0.07), [2] |
2 (0.07), [2] |
|
Neutropenia |
1 (0.03), [1] |
- |
|
Musculoskeletal disorder |
2 (0.07), [2] |
- |
|
Chest muscular pain |
1 (0.03), [1] |
- |
|
Rhabdomyolysis |
1 (0.03), [1] |
- |
|
Metabolic and nutrition disorder |
2 (0.07), [2] |
1 (0.03), [1] |
|
Anorexia |
1 (0.03), [1] |
1 (0.03), [1] |
|
Hypokalemia |
1 (0.03), [1] |
- |
|
Neurologic disorder |
1 (0.03), [1] |
- |
|
Sensory loss |
1 (0.03), [1] |
- |
|
Laboratory abnormality |
2 (0.07), [2] |
2 (0.07), [2] |
|
LFT abnormality |
2 (0.07), [2] |
2 (0.07), [2] |
|
Skin and soft tissue disorder |
2 (0.07), [2] |
- |
|
Itching sense |
1 (0.03), [1] |
- |
|
Drug eruption |
1 (0.03), [1] |
- |
|
Vascular disorder |
1 (0.03), [2] |
- |
|
Hypertension |
1 (0.03), [1] |
- |
|
Hypotension |
1 (0.03), [1] |
- |
|
Cardiac disorder |
1 (0.03), [1] |
- |
|
Tachycardia |
1 (0.03), [1] |
- |
|
Total |
35 (1.16), [42] |
10 (0.33), [11] |

The incidence rate of AEs of the persons 65 years or older was 3.84% (14/365 persons), which was significantly higher than persons under 65 years old (0.79%, 21/2,659) (P < 0.001). AEs were more common in the subjects with underlying diseases (3.87% vs. 0.53%, P < 0.001). Subjects with concomitant medications had more AEs than the others (1.56% vs. 0.22%, P = 0.002). ADRs were also more common in the subjects with concomitant medication (0.47% vs. 0%, P = 0.038).
The results of the logistic regression analysis of the AEs incidence showed that AEs occurred more frequently in the subjects with influenza complications (odds ratio [OR], 3.949; 95% CI, 1.877–8.308), underlying diseases (OR, 7.555; 95% CI, 3.782–15.091), or concomitant medications (OR 7.252; 95% CI, 1.737–30.274). The results of the logistic regression analysis of ADRs incidence showed that the ADRs occurred more frequently in the subjects with underlying diseases (OR, 4.153; 95% CI, 1.068–16.148).
Safety assessment in special groups
Seven pregnant women were included in the study, but no AE was identified. Forty-six patients with underlying liver disease were included in the study. Among them, pneumonia was detected in 2.17% (1/46, 1 subject) as an AE, but there was no ADR. Four patients with underlying kidney disease were included in the study, but no AE was identified.
Effectiveness assessment
After administration of peramivir, the severity of influenza symptoms decreased by 10.68 ± 4.01 points and the daily living performance was improved by 5.59 ± 2.16 points (
Table 3). In both effectiveness assessments, pre- and post-treatment differences were statistically significant (
P < 0.001).
Table 3
Change of the severity of influenza symptoms and the daily living performance after the administration of peramivir

|
Characteristics |
The severity of influenza symptom |
The daily living performance |
|
No. |
Average ± SD |
Median |
Range |
No. |
Average ± SD |
Median |
Range |
|
Before |
2,758 |
12.61 ± 4.39 |
13.00 |
0.00–21.00 |
2,712 |
3.47 ± 2.08 |
3.00 |
0.00–10.00 |
|
After |
2,638 |
2.12 ± 2.27 |
2.00 |
0.00–17.00 |
2,619 |
9.04 ± 1.46 |
10.00 |
0.00–10.00 |
|
Change |
2,634 |
−10.68 ± 4.01 |
−11.00 |
−21.00–3.00 |
2,613 |
5.59 ± 2.16 |
6.00 |
−8.00–10.00 |
|
Rate of change |
2,633 |
−84.11 ± 16.19 |
−86.67 |
−100.00–42.86 |
2,558 |
257.08 ± 225.82 |
200.00 |
−100.00–900.00 |
|
P value |
< 0.001 |
< 0.001 |

In the sub-analysis of the changes of the severity of influenza symptoms, the females showed more decrease than the males (10.83 ± 3.88 vs. 10.44 ± 4.20, P = 0.028). Adults younger than 65 years showed more decrease than the older adults (10.95 ± 3.84 vs. 8.56 ± 4.61, P < 0.001). Subjects with influenza complication showed less decrease than those without complication (9.29 ± 3.49 vs. 10.83 ± 4.04, P < 0.001). Subjects with underlying diseases showed less decrease than the others (8.40 ± 4.72 vs. 11.19 ± 3.64, P < 0.001). In the sub-analysis of the changes of the daily living performance, there was no statistically significant difference according to the subjects' characteristics.
Influenza-related symptoms disappeared on average 3.02 ± 2.39 days after peramivir administration (median 2.00 days, range 0–32 days). The persons 65 years or older took more days to lose influenza symptoms than the younger persons (3.55 ± 2.80 vs. 2.95 ± 2.32, P < 0.001). Subjects with underlying diseases took more days than the others (3.82 ± 2.68 vs. 2.83 ± 2.28, P < 0.001). Subjects with influenza B took more days than those with influenza A (3.17 ± 2.31 vs. 2.96 ± 2.41, P < 0.001).
The results of the final effectiveness assessment as follows: effective, 97.96% (2,879/2,939); unable to judge, 1.02% (30/2,939); not done, 0.75% (22/2,939); and no effective, 0.27% (8/2,939).
DISCUSSION
Peramivir has been approved for emergency use during the A/H1N1pdm09 pandemic prior to formal licensing.
7 At that time, peramivir was usually used as a salvage treatment in severe patients who had no effect on oseltamivir. There were a few reports on the safety and effectiveness during emergency use and the results were acceptable for clinical use.
8910 After that, the result of the phase III clinical trial was reported and the peramivir with once daily regimen showed acceptable safety and effectiveness compared to the 5-day regimen of oral oseltamivir.
5 However, since peramivir was approved only in a few countries, such as Japan, Korea, and the United States, safety and effectiveness data in clinical use was relatively limited. Most of the studies were conducted in Japan and showed similar effectiveness and safety compared with oseltamivir.
111213 There are two clinical studies of peramivir in Korea that are searchable through PubMed. One study compared the clinical effectiveness of oral oseltamivir and intravenous peramivir in patients with severe influenza.
14 Both antiviral agents showed similar clinical effectiveness. Another study was a meta-analysis, not a direct clinical study.
15 Although this study was conducted as a post-marketing surveillance, it has strength in terms of a prospective clinical study with more than 3,000 subjects during various influenza seasons. It is also meaningful in that the safety and effectiveness of peramivir are evaluated with a variety of subjects including healthy adults, the elderly and patients with chronic diseases.
Compared with the phase III clinical trial
5 and the post-marketing surveillance conducted in Japan,
111213 the incidence of AEs identified in this study was much lower. This may be due to differences in the characteristics of the population, or due to the differences in the subjects' perception of the AEs. However, there was no difference in that most AEs were mild and the SAE was rare.
In this study, AEs of peramivir were found to be more frequent in subjects with influenza complications, underlying diseases, or concomitant medications. This is thought to be due to the underlying condition of subjects rather than the peramivir drug itself.
Two deaths were identified during this study. One case was a patient admitted to the emergency room accompanied by influenza and severe pneumonia, and died two days after receiving peramivir. Another case was a patient with alcoholic liver disease, non-Hodgkin's lymphoma, cardiomyopathy and diabetes mellitus, and died two days after receiving peramivir with severe pneumonia. In both cases, the death was possibly related to complicated pneumonia following influenza rather than the AE of peramivir. However, since the peramivir is likely to be administered to severe patients due to the possibility of intravenous use, a large-scale prospective study will be needed to analyze the effectiveness of peramivir compared to other antiviral agents in patients with severe influenza.
Seven pregnant women were included in this study. Peramivir is generally not recommended for pregnant women in the guideline due to the insufficient safety data.
3 The pregnant women were monitored until the birth results to be confirmed, and there was no specific problem related to the birth. However, the results of this study are not enough to confirm the safety of peramivir in pregnant women. Therefore, it cannot be recommended to pregnant women with this data. Additional research and data collection will be needed to confirm the safety of peramivir during pregnancy.
The effectiveness of peramivir was confirmed by a significant improvement in influenza symptom severity and daily living performance before and after administration of peramivir. However, since this study was a single-arm study in which placebo or other antiviral agents were not used as a control group, it was impossible to confirm the efficacy compared to the placebo or other antiviral agents. Sub-analysis showed better effectiveness in patients with young age, without influenza complication, or without underlying disease. This result is considered to reflect the underlying condition of subjects rather than the pharmacodynamic properties of peramivir. It is not clear why the influenza symptom severity was significantly improved in females compared to males. One of the possible explanations is that men are likely to state symptoms with narrow spectrum due to cultural factors. It is not clear why the symptom of influenza B disappeared later than influenza A. Further studies will be required to evaluate the effectiveness of peramivir in each type of influenza virus.
More than 1,000 subjects were excluded from the analysis. Of those, 357 had no laboratory confirmation of influenza infection. However, in the current guidelines for the use of influenza antiviral agents, high-risk patients are recommended to receive empirical antiviral agents during influenza epidemics without laboratory confirmation of influenza virus infection. Supplementary analysis was performed with the data of excluded subjects. Among the 950 subjects with sufficient data, the incidence of AE was 2.53% (24/950), the incidence of ADR was 0.32% (3/950), and the incidence of SAE was 0.42% (4/950). There was no significant difference compared to the subjects included in the main analysis.
This study has some limitations. Because this study was conducted according to the regulation of the Republic of Korea, research design and data collection were limited in comparison with other clinical studies. This was a single-arm study that cannot compare the safety and effectiveness with placebo or other antiviral agents. The number of excluded subjects was relatively large, which was about 25% of the total enrolled subjects. The data of children was excluded from the analysis because peramivir is approved only for adults in Korea.
In conclusion, peramivir was confirmed to have a tolerable safety profile and acceptable effectiveness in Korean adult patients with seasonal influenza, which was similar to those of previous clinical studies.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download