INTRODUCTION
Invasive bacterial infections are a major cause of morbidity and mortality in children.
Streptococcus pneumoniae,
Haemophilus influenzae,
Neisseria meningitidis,
Streptococcus agalactiae,
Streptococcus pyogenes,
Staphylococcus aureus,
Salmonella species,
Listeria species, and
Escherichia coli are major bacterial pathogens that are responsible for invasive infections in immunocompetent children.
12 However, the main organisms causing various clinical syndromes vary by age and over time. Thus, empirical antimicrobial therapy of suspected bacterial infections must be tailored to the common etiological agents of the infections in each region. In addition, countries employ immunization programs by prioritizing the burden of diseases.
To estimate the burden of invasive bacterial infections, many countries establish a nationwide surveillance system, such as Active Bacterial Core surveillance by the Centers for Disease Control and Prevention in the United States
3 and Health Protection Agency surveillance in the United Kingdom.
4 Japan and Taiwan have also nationwide surveillance systems for invasive bacterial infections.
56 Although Korea launched a surveillance system for invasive bacterial infections that are preventable by the national immunization program such as diphtheria, pertussis, tetanus, and
H. influenzae type b, this surveillance system did not include the most prevalent pathogens. Additionally, the notification rate remained low; 17.4%–43.0% for vaccine preventable diseases in the 2000s.
78
A retrospective study of invasive bacterial infections in Korean children at 18 university hospitals across the country was conducted over a ten-year period from 1996 to 2005.
9 The study was the first multicenter, hospital-based surveillance study conducted in Korea. In the study,
S. pneumoniae was found to be responsible for 44% (123 of 279 cases) of invasive bacterial diseases in immunocompetent children 3 to 59 months of age. Following this study, a remarkable change in immunization practices occurred to prevent invasive pneumococcal diseases (IPDs), which are the leading cause of bacterial infections in children. The pneumococcal conjugate vaccine 7 (PCV7) was introduced at the end of 2003, and the PCV7 was replaced by the PCV10 and PCV13 in 2010. This time period, after PCV7 use and before PCV13 introduction, provides data on the epidemiological changes of invasive bacterial infections influenced by PCV7 use.
Thus, we conducted a retrospective, multicenter study to analyze the relative proportion of the major bacterial agents responsible for invasive infections in apparently immunocompetent Korean children from 2006 to 2010 according to age, clinical features, and time. In addition, this study was conducted as a continuance of the previous multicenter surveillance study performed from 1996–2005,
9 permitting a comparison of data from the two study periods.
RESULTS
Etiology
A total of 947 episodes of invasive bacterial infections and 950 bacterial organisms were identified during the study period. Co-infections were observed in 3 episodes. The male:female ratio was 0.6:1. Regardless of age and clinical diagnosis, S. aureus was the most frequently isolated organism (n = 317, 33.5%), followed by E. coli (n = 192, 20.3%), S. pneumoniae (n = 174, 18.4%), and S. agalactiae (n = 133, 14.4%). Salmonella species and S. pyogenes accounted for 67 cases (7.1%) and 25 cases (2.6%), respectively. There were very few cases of infection with H. influenzae (18 cases), N. meningitidis (14 cases), or L. monocytogenes (4 cases).
Of 67 Salmonella isolates, serogroup data were available for 62. Among these, 14 isolates were Salmonella typhi and 48 isolates were non-typhoidal Salmonella which included Salmonella paratyphi (n = 14), non-typhoidal group D (n = 9), serogroup A (n = 1), serogroup B (n = 4), serogroup C (n = 28), serogroup E (n = 1), and serogroup G (n = 1).
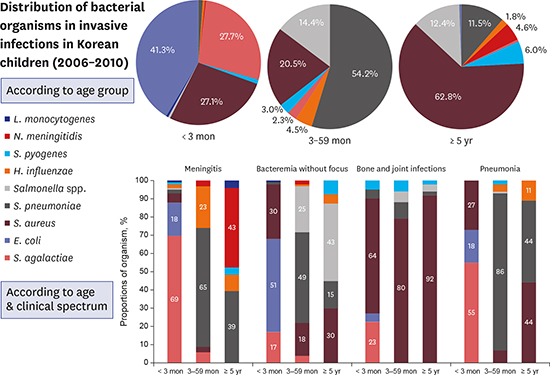
Distribution of causative bacterial organisms by age
The age-related distribution of causative bacterial organisms is shown in
Table 1. The number of cases was highest in < 3 months of age group, with 465 cases (49%), followed by ≥ 5 years of age group with 218 cases (23%), the 24–59 months of age group with 134 cases (14%), and the 3–23 months of age group with 130 cases (14%). Patients < 5 years of age accounted for 76% of all cases. The distribution of bacteria differed by age group.
E. coli was the most prevalent organism among infants < 3 months of age and accounted for 41.3% of the cases in this age group, followed by
S. agalactiae and
S. aureus, which accounted for 27.7% and 27.1% of cases, respectively. These three organisms were responsible for 96.1% of all the invasive bacterial infections in infants < 3 months of age.
Table 1
Distribution of causative bacterial organisms among 947 invasive infections from 2006 to 2010, by age group

|
Identified species of bacteria |
Age group, No. (%) |
Total |
|
< 3 months |
3–59 months |
≥ 5 years |
|
3–23 months |
24–59 months |
Subtotal |
|
S. aureus
|
126 (27.1) |
30 (23.1) |
24 (17.9) |
54 (20.5) |
137 (62.8) |
317 (33.5) |
|
S. pneumoniae
|
6 (1.3) |
66 (50.8) |
77 (57.5) |
143 (54.2) |
25 (11.5) |
174 (18.4) |
|
S. agalactiae
|
129 (27.7) |
6 (4.6) |
0 (0.0) |
6 (2.3) |
1 (0.5) |
136 (14.4) |
|
Salmonella spp. |
2 (0.4) |
17 (13.1) |
21 (15.7) |
38 (14.4) |
27 (12.4) |
67 (7.1) |
|
H. influenzae
|
2 (0.4) |
5 (3.8) |
7 (5.2) |
12 (4.5) |
4 (1.8) |
18 (1.9) |
|
S. pyogenes
|
4 (0.9) |
3 (2.3) |
5 (3.7) |
8 (3.0) |
13 (6.0) |
25 (2.6) |
|
N. meningitidis
|
1 (0.2) |
3 (2.3) |
0 (0.0) |
3 (1.1) |
10 (4.6) |
14 (1.5) |
|
L. monocytogenes
|
3 (0.6) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (0.5) |
4 (0.4) |
|
E. coli
|
192 (41.3) |
NAa
|
NA |
NA |
NA |
192 (20.3) |
|
Total |
465 |
130 |
134 |
264 |
218 |
947 |

S. pneumoniae, S. aureus, and Salmonella species were isolated frequently from infants and children ≥ 3 months of age. S. pneumoniae was the most common bacterial agent responsible for invasive infections in infants and children 3 to 59 months of age, accounting for 54.2% (143/264) of cases. In contrast, S. pneumoniae was the third most common cause of bacterial infections in children ≥ 5 years of age (11.5% of cases). S. aureus was a significant cause of infections in children 3 to 59 months of age, accounting for 20.4% of invasive infections that developed in this age group, and was the leading cause of bacterial infection in children ≥ 5 years of age (62.8% of cases). Salmonella species accounted for 13.5% of invasive infections in children ≥ 3 months of age.
Distribution of etiologic organisms according to clinical diagnosis
Among the 947 invasive bacterial infections identified, bacteremia without localizing signs was most common, accounting for 48.9% (n = 463) of cases, followed by arthritis and osteomyelitis (n = 163, 17.2%), meningitis (n = 141, 14.9%), and pneumonia with bacteremia or empyema (n = 113, 11.9%). Other diseases not assigned to one of the above clinical entities accounted for 7% of cases.
The distributions of the causative organisms according to three age groups (< 3 months of age, 3 to 59 months of age, and ≥ 5 years of age) for the 4 most common clinical diagnoses are illustrated in
Fig. 2. For meningitis, most cases were attributed to
S. agalactiae (69.0%) in patients < 3 months of age. In patients 3 to 59 months of age,
S. pneumoniae (65.0%) and
H. influenzae (23.0%) were frequently identified in meningitis cases.
N. meningitidis (43.0%) and
S. pneumoniae (39.0%) caused most of the meningitis cases in patients ≥ 5 years of age. For bacteremia without localizing signs,
E. coli (51%),
S. aureus (30.0%), and
S. agalactiae (17.0%) were the predominant causes of infection among infants < 3 months of age. In contrast,
S. pneumoniae was the leading cause of bacteremia without localizing signs in 3 to 59 months of age group (49.0%), followed by
Salmonella species (25.0%) and
S. aureus (18.0%).
Salmonella species (43.0%) and
S. aureus (30.0%) were common causes of bacteremia in children ≥ 5 years of age.
 | Fig. 2Distribution of the causative bacterial organisms in invasive infections in immunocompetent Korean children between 2006 and 2010 according to age group and clinical spectrum. 
|
S. aureus was the most prevalent cause of bone and joint infections in all the age groups, causing 64.0% of cases in < 3 months of age group, 80.0% of cases in the 3–59 months of age group, and 92.0% of cases in ≥ 5 years of age group. For pneumonia, S. agalactiae predominated in < 3 months of age group. In the other age groups, however, S. pneumoniae was the most frequent cause of infection, responsible for 86.0% of cases in 3 to 59 months of age group and 44.0% of cases in ≥ 5 years of age group. S. aureus showed the same proportion as S. pneumoniae in ≥ 5 years of age group.
Changes in the relative proportion of bacterial organisms over time
Data from the previous 10-year study of 1996–2005
9 were reconstructed for comparison of the distributions of the bacterial organisms according to age group. Distributions of the relative proportions of the 8 bacteria that caused invasive infections in < 3 months of age group are shown in
Table 2. For an exact comparison with the previous study period,
E. coli, which was surveyed only in our study, was excluded and recalculated. The study periods were divided into 5-year increments: 1996–2000, 2001–2006, and 2006–2010.
S. aureus and
S. agalactiae were the two most prevalent organisms throughout the collective study period. When we performed the trend comparisons between the three five-year periods, no significant changes in the organismal trends were observed, except for
H. influenzae. Over 15 years, there was a decreasing trend in the relative proportion of
H. influenzae < 3 months of age group (
P = 0.036).
Table 2
Distribution in the relative proportions of the 8 causative organisms of invasive infections

|
Species of bacteriaa
|
Period, No. (%) |
P-value |
|
1996–2000 |
2001–2005 |
2006–2010 |
|
S. aureus
|
35 (34.0) |
71 (39.0) |
126 (46.2) |
0.078 |
|
S. pneumoniae
|
2 (1.9) |
9 (4.9) |
6 (2.2) |
0.901 |
|
S. agalactiae
|
49 (47.6) |
88 (48.4) |
129 (47.3) |
0.966 |
|
Salmonella spp. |
4 (3.9) |
1 (0.5) |
2 (0.7) |
0.080 |
|
H. influenzae
|
6 (5.8) |
5 (2.7) |
2 (0.7) |
0.036 |
|
S. pyogenes
|
3 (2.9) |
7 (3.8) |
4 (1.5) |
0.544 |
|
N. meningitidis
|
2 (1.9) |
0 (0.0) |
1 (0.4) |
0.224 |
|
L. monocytogenes
|
2 (1.9) |
1 (0.5) |
3 (1.1) |
0.598 |
|
Total |
103 |
182 |
273 |
|

The temporal changes in the relative proportions of the 8 most prevalent bacterial organisms in children ≥ 3 months of age between 1996 and 2010 were analyzed (
Fig. 3). There was a significantly increasing trend of
S. aureus infections over these 15 years (
P < 0.001). In 1996, the relative proportion of
S. aureus infections was 13.8%, which rose to 42.2% in 2006 and 38.6% in 2010.
H. influenzae infections continued to show a significantly decreasing trend (
P < 0.001). The relative proportion of
H. influenzae infections in 1996 was 41.4%, and in 2010, it was 4.5%. No significant changes in the relative proportions of infections with
S. pneumoniae and
Salmonella species were observed.
 | Fig. 3Temporal changes in the relative proportions of the 8 most important bacterial organisms of invasive infections in immunocompetent Korean children 3 months of age or older between 1996 and 2010. 
|
Clinical outcomes
Clinical outcomes were available for 877 (92%) of the 947 cases; 70 cases (8%) had missing data due to ‘transferred to another hospital’ or ‘discharged against medical advice.’ The overall case-fatality rate was 2.5% (22/877). When the data were analyzed according to clinical diagnosis, meningitis had the highest case-fatality rate (10.2%, 13/127), followed by bacteremia without localizing signs (2.0%, 6/301) and pneumonia (1.9%, 2/105). When the data were analyzed according to age groups, the 3–23 months of age group had the highest case-fatality rate (8.6%, 10/116), followed by the 24–59 months of age group (2.4%, 3/124), the infants < 3 months of age group (18%, 8/437), and ≥ 5 years of age group (0.5%, 1/200).
Among the bacteria studied, N. meningitidis was associated with the highest case-fatality rate of 9.1% (1/11); however, the overall number of N. meningitidis infections was small. When bacteria that were isolated in 50 or more cases were analyzed, we found that S. pneumoniae had the highest case-fatality rate of 8.4% (13/155), followed by S. agalactiae with a case fatality rate of 4.8% (6/126). S. pyogenes and E. coli had case fatality rates of 4.2% (1/24) and 0.5% (1/185), respectively. No fatalities associated with H. influenzae infection were identified during the study period.
In terms of the frequency of sequelae associated with meningitis cases, H. influenzae had a very high sequelae rate of 50% (4/8). Sequelae rates associated with meningitis caused by other common infective organisms, namely, S. pneumoniae, S. agalactiae, and E. coli, showed similar trends compared to their case-fatality rates and were 36.8% (7/19), 30.8% (16/52), and 13.3% (2/15), respectively. The most common sequelae were hydrocephalus, subdural effusion, and hearing loss. Long-term sequelae such as developmental delay and cognitive impairment were not evaluated due to the retrospective design of the study.
DISCUSSION
The purpose of this study was to investigate the etiology of invasive bacterial infections in a pediatric population in Korea through a retrospective review of multicenter hospital-based surveillance. This study is important not only for its purpose but also for its continuous maintenance of multicenter-based surveillance since 1996. The study period between 2006 and 2010 corresponds to the period after PCV7 use and before PCV13 introduction in Korea. Data collected during this period reflect the epidemiological changes in invasive bacterial infections that were influenced by PCV7 use. Additionally, the number of cases (n = 947) and bacterial organisms (n = 950) included in this study comprise the largest invasive infection study in Korea to date. Nationwide or regional data collected during a certain time period can be used to reliably analyze the major pathogens responsible for invasive bacterial infections. Twenty eight percent (25/89) of university hospitals in Korea participated in this study design, and they were distributed nationwide. The data in the current study will be useful for planning the empirical treatment of invasive bacterial infections as well as for establishing a national vaccine policy.
Some notable findings in this study are as follows. First, an expanded number of hospitals and clinical episodes were included in the current study compared to the 1996–2005 period: 18 hospitals and 768 episodes comprised the 1996–2005 study, while 25 hospitals and 947 episodes were included in the 2006–2010 period study. Second, we added our data to those of the previous 10 years. Thus, we were able to compare the relative proportions and trends of the 8 bacteria in both < 3 months of age group and ≥ 3 months of age groups over 15 years, from 1996 to 2010. During this period, S. aureus showed a significantly increasing trend in children ≥ 3 months of age. Third, H. influenzae continued to show a decreasing trend in both < 3 months of age group and ≥ 3 months of age groups.
In our study, the major pathogens that caused invasive bacterial infections differed according to age: for patients < 3 months of age, E. coli, S. agalactiae, and S. aureus predominated; for patients 3 to 59 months of age, S. pneumoniae, S. aureus, and Salmonella species were most prevalent; and for patients ≥ 5 years of age, S. aureus, Salmonella species, and S. pneumoniae dominated. In neonates and infants < 3 months of age, S. agalactiae was a major cause of meningitis, and E. coli was a major cause of bacteremia. In children ≥ 3 months of age, S. pneumoniae caused pneumonia, bacteremia, and meningitis. S. aureus was the main cause of bone and joint infections and bacteremia in this age group.
S. aureus has a diverse clinical spectrum that ranges from asymptomatic colonization, to skin and soft tissue infection, and to bone and joint infections.
S. aureus is a major neonatal pathogen in Korea.
11 Additionally,
S. aureus is becoming an increasingly frequent cause of community-acquired pneumonia in children < 5 years of age.
12 Up to 80% of bone and joint infections in children are caused by
S. aureus. Generally, the relative proportions of the causative organisms in the different age groups and clinical diagnoses in our study were consistent with earlier studies. One notable finding was the antimicrobial sensitivity of the
S. aureus isolates. We found that 38.7% of all the
S. aureus isolates were oxacillin resistant, which is a higher proportion than those reported by previous studies.
13
S. agalactiae remains one of major pathogens responsible for neonatal invasive infections in developed countries.
1415 In Korea, neonatal infections caused by
S. agalactiae have increased in recent years.
16 In our findings, the relative proportion of invasive infections in infants < 3 months of age is consistent with previous studies. In a study by Lee et al.,
2 S. agalactiae accounted for 38% of the invasive infections in infants < 3 months of age. In the same age group,
S. agalactiae accounted for 48.1% of the invasive infections in a previous study conducted from 1996 to 2005.
9 In this study, from 2006 to 2010,
S. agalactiae was the most prevalent organism, accounting for 47.3% of the infections. The exclusion of
E. coli to allow for comparison with the previous study yielded this result. The previous 1996–2005 period study did not include
E. coli; this study showed that
E. coli was the most common pathogen to cause invasive bacterial infections in infants < 3 months of age.
E. coli mainly caused bacteremia without localizing signs, or meningitis in this age group. Siitonen et al.
17 also reported that the common infections caused by
E. coli were bacteremia, pyelonephritis, and meningitis in a Finnish study performed during the late 1980s. The case-fatality rate caused by
E. coli during the neonatal period was 9%. In the current study, the case-fatality rate of
E. coli infection was 0.5% (1 of 185 identifiable cases). Although the case fatality rate was relatively low compared to the other organisms,
E. coli is still a major invasive pathogen in neonates and infants < 3 months of age in terms of the relative proportion.
S. pneumoniae was the most common pathogen to cause invasive bacterial infections among children 3 to 59 months of age, accounting for 45.3% of infections from 1996–2006 in Korea.
9 In our study, 54.4% of the invasive infections were caused by
S. pneumoniae, which was the most prevalent pathogen of bacteremia without localizing signs, meningitis, and pneumonia in the same age group. The reduction of invasive infections by
H. influenzae and
Salmonella species resulted in this high proportion of
S. pneumoniae in children 3 to 59 months of age.
S. pneumoniae also had the highest case-fatality rate regardless of age in the current study. After the introduction of PCV7 in 2000 and of PCV13 in 2010 in the United States, the overall incidence of IPD declined from 100 cases per 100,000 children under 5 years of age in 1998 to 9 cases per 100,000 in 2015. IPD caused by PCV13 serotypes declined from 91 cases per 100,000 children under 5 years of age in 1998 to 2 cases per 100,000 in 2015.
18 In Korea, PCV7 was offered as an optional vaccine in 2003, and vaccine coverage reached up to 70% in 2009. The proportion of IPD in children 3 to 59 months of age in this study period, 2006–2010, was 54.2%, and it was 39.6% in the 1996–2000 period and 45.7% in the 2001–2005 period. This increase in the relative proportion of IPD might have simply gained from reduction of other bacterial organisms. Another partial explanation given is that due to low PCV7 uptake rate during the first half of this study period and changes in the serotype distribution among IPD cases, a decrease in the proportion of IPD was not observed in this study period. Although PCV7 was introduced at the end of 2003, the vaccination rate was low for several years and the vaccine uptake rate of the primary 3 dose series reached around 60% in 2007.
19 According to data from a study performed during the same time period, 2006–2010, the proportion of PCV7 serotypes decreased from 62.5% in 2006 to 21.4% in 2010, whereas the proportion of PCV13-specific serotypes, especially 19A, increased from 17.4% in 2006 to 47.8% in 2010.
20 Therefore, the proportion of PCV7 serotypes decreased while PCV13-specific serotypes increased during the 2006–2010 period, reducing the impact of PCV7 and making
S. pneumoniae still one of the most important pathogens of invasive infections. Analysis of the relative proportion of IPD after the 2006–2010 period will be available in a follow-up study that will include the period after 2010, when the PCV10 and PCV13 were introduced, as well as the period after 2014, when PCVs were implemented in the national immunization program in Korea.
Before the introduction of conjugate vaccines,
H. influenzae was the major pathogen that caused meningitis, pneumonia, epiglottitis, arthritis, and cellulitis worldwide.
2122 H. influenzae infection has transitioned, however, from a major cause of childhood illness to a rare disease in every country where conjugate vaccines have been introduced into the routine immunization schedule.
23 In Korea, an
H. influenzae vaccine was introduced in 1996 and was implemented in the routine immunization schedule in 2013. In a study performed in the pre-vaccination era, Nam and Lee
1 reported that
H. influenzae was a major pathogen, accounting for 14% of all bacterial infections, 88% of which were reported to be meningitis. In a previous study by Lee et al.,
9 H. influenzae infections accounted for 20.1% of all invasive infections and 54.2% of meningitis infections in children ≥ 3 months of age during the first half of the study period, from 1996 to 2000. However, this proportion dropped to 4.5% of all invasive infections and 19.7% of all meningitis infections in the latter half of the study period, from 2001 to 2005. In our study, we observed a significant decline in all of the age groups over the 15-year period, from 1996 to 2010.
Salmonella species are water- and food-borne pathogens in low-resource countries. The risk for infection is high in countries with poor sanitation and lack of access to safe food and water. Meanwhile, invasive
Salmonella infections have declined in countries where the treatment of municipal water, pasteurization of dairy products, and improvement of public sanitation have been introduced.
24 In the current and previous studies,
Salmonella infections have constantly declined: 23% in the 1986–1995 period, 10% in the 1996–2005 period, and 7% in the 2006–2010 period.
19 However, 13.5% of the infections in children ≥ 3 months of age were attributed to
Salmonella species. In the past, most cases of salmonellosis were associated with
S. typhi; in the present, with the increase in the consumption of meat, infections due to nontyphoidal
Salmonella occur consistently.
25 In our study,
Salmonella species were most often isolated from cases of bacteremia without localizing signs in ≥ 3 months of age groups, which was consistent with the findings of the prior study.
This study had several limitations. We targeted only nine bacterial organisms. However, by focusing on these nine bacterial organisms, most of the invasive bacterial infections in the immunocompetent children were likely included in our analysis. These nine bacterial organisms are the most important pathogens that cause infections in children who do not have any underlying diseases or conditions related to an immune-compromised state. Different numbers of hospitals were included in the current and present study. This may have affected the result of trend analysis in making comparisons across study periods. Hospitals included in the 15-year study period, however, are university-affiliated hospitals and supposed to have similar characteristics. The retrospective design of the study may have resulted in bias. Cases of mild illness such as occult bacteremia, where the attending doctor might not perform cultures for out-patients, were excluded from this study and thus may have introduced a selection bias. Variations in laboratory protocols for the detection of bacteria in the participating hospitals could also have affected our results. For a more acute description of the distribution and etiology of invasive bacterial infections in Korea, further well-designed prospective studies are needed. This study, however, collected invasive bacterial infection cases steadily over 15 years in the absence of nationwide surveillance. The data in this study can provide epidemiological data of invasive bacterial infections in Korean children and serve as a means to judge the effects of vaccine policies.
In conclusion, the distributions of bacteria that cause invasive infections differed by age group and clinical diagnosis. In children ≥ 3 months of age, S. pneumoniae remained an important pathogen due to its incidence and fatality rate. The relative proportion of S. aureus increased significantly over the 15-year study period in children ≥ 3 months of age. Over the same time period, the relative proportion of invasive infections caused by H. influenzae showed a decreasing trend in both < 3 months of age group and ≥ 3 months of age groups. The national immunization policy may lead to changes in the etiology of invasive bacterial infections. Additionally, epidemics of certain bacterial infections may emerge unexpectedly. Thus, multicenter surveillance studies similar to ours should be continuously performed in the future.







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download