INTRODUCTION
METHODS
Patient selection
Interpretation of pathologic diagnosis and histologic subtypes of IPNB
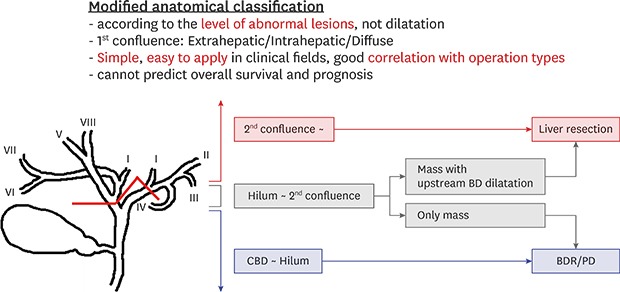
Radiologic characteristics of IPNB and anatomical and morphological classifications
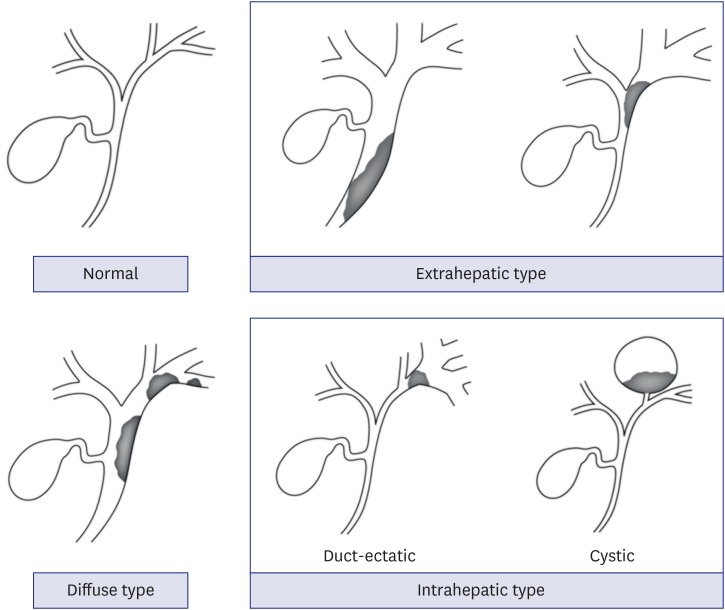
Extrahepatic type: the main lesions were confined to the common bile duct and common hepatic duct
Intrahepatic type: the main lesions were located at the periphery beyond the first confluence of the IHD
Diffuse type: the main lesions were located over a wide range of the IHD and EHD
Correlation between the clinicopathologic characteristics of IPNB and anatomical and morphological classifications
Statistical analysis
RESULTS
Demographic findings
Table 1
Patients' characteristics
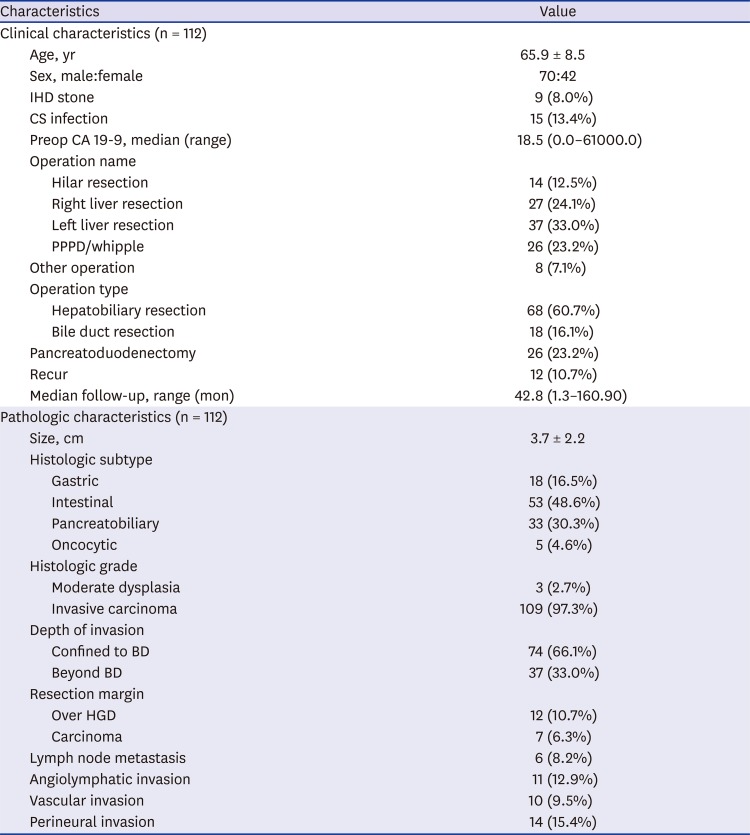
Pathologic findings
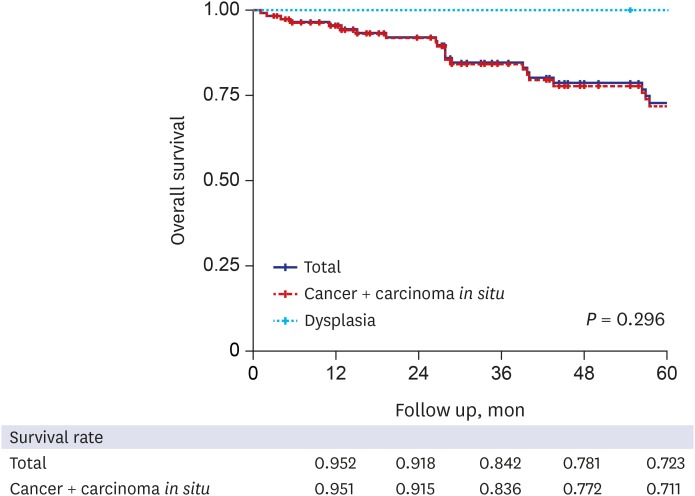
Survival outcome and prognostic factors
Fig. 3
Overall survival according to the various prognostic factors. (A) Overall survival according to the histologic subtypes. (B) Overall survival according to the JBA classification.10 (C) Overall survival according to the classification by Kim et al.9 (D) Overall survival according to the modified anatomical classification. (E) Overall survival according to the resection margin status. (F) Overall survival according to the lymph node metastasis.
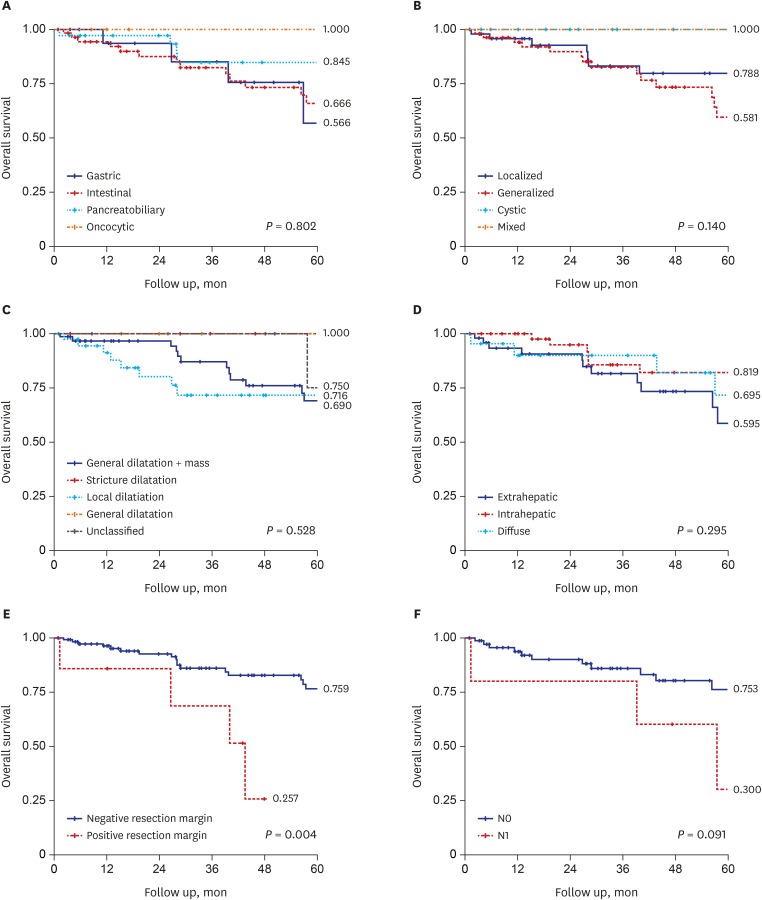
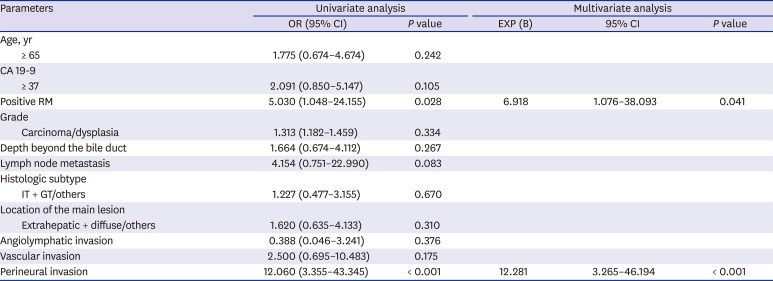
Table 2
Prognostic factors for survival
Radiologic characteristics according to various morphological and anatomical classifications based on preoperative CT
Table 3
Clinicopathologic characteristics according to morphological and anatomical classifications
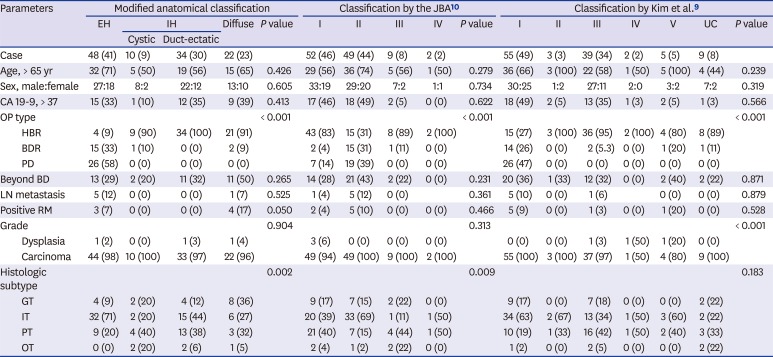
| Parameters | Modified anatomical classification | Classification by the JBA10 | Classification by Kim et al.9 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EH | IH | Diffuse | P value | I | II | III | IV | P value | I | II | III | IV | V | UC | P value | |||
| Cystic | Duct-ectatic | |||||||||||||||||
| Case | 48 (41) | 10 (9) | 34 (30) | 22 (23) | 52 (46) | 49 (44) | 9 (8) | 2 (2) | 55 (49) | 3 (3) | 39 (34) | 2 (2) | 5 (5) | 9 (8) | ||||
| Age, > 65 yr | 32 (71) | 5 (50) | 19 (56) | 15 (65) | 0.426 | 29 (56) | 36 (74) | 5 (56) | 1 (50) | 0.279 | 36 (66) | 3 (100) | 22 (58) | 1 (50) | 5 (100) | 4 (44) | 0.239 | |
| Sex, male:female | 27:18 | 8:2 | 22:12 | 13:10 | 0.605 | 33:19 | 29:20 | 7:2 | 1:1 | 0.734 | 30:25 | 1:2 | 27:11 | 2:0 | 3:2 | 7:2 | 0.319 | |
| CA 19-9, > 37 | 15 (33) | 1 (10) | 12 (35) | 9 (39) | 0.413 | 17 (46) | 18 (49) | 2 (5) | 0 (0) | 0.622 | 18 (49) | 2 (5) | 13 (35) | 1 (3) | 2 (5) | 1 (3) | 0.566 | |
| OP type | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||
| HBR | 4 (9) | 9 (90) | 34 (100) | 21 (91) | 43 (83) | 15 (31) | 8 (89) | 2 (100) | 15 (27) | 3 (100) | 36 (95) | 2 (100) | 4 (80) | 8 (89) | ||||
| BDR | 15 (33) | 1 (10) | 0 (0) | 2 (9) | 2 (4) | 15 (31) | 1 (11) | 0 (0) | 14 (26) | 0 (0) | 2 (5.3) | 0 (0) | 1 (20) | 1 (11) | ||||
| PD | 26 (58) | 0 (0) | 0 (0) | 0 (0) | 7 (14) | 19 (39) | 0 (0) | 0 (0) | 26 (47) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Beyond BD | 13 (29) | 2 (20) | 11 (32) | 11 (50) | 0.265 | 14 (28) | 21 (43) | 2 (22) | 0 (0) | 0.231 | 20 (36) | 1 (33) | 12 (32) | 0 (0) | 2 (40) | 2 (22) | 0.871 | |
| LN metastasis | 5 (12) | 0 (0) | 0 (0) | 1 (7) | 0.525 | 1 (4) | 5 (12) | 0 (0) | 0.361 | 5 (10) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0.879 | |||
| Positive RM | 3 (7) | 0 (0) | 0 (0) | 4 (17) | 0.050 | 2 (4) | 5 (10) | 0 (0) | 0 (0) | 0.466 | 5 (9) | 0 (0) | 1 (3) | 0 (0) | 1 (20) | 0 (0) | 0.528 | |
| Grade | 0.904 | 0.313 | < 0.001 | |||||||||||||||
| Dysplasia | 1 (2) | 0 (0) | 1 (3) | 1 (4) | 3 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 1 (50) | 1 (20) | 0 (0) | ||||
| Carcinoma | 44 (98) | 10 (100) | 33 (97) | 22 (96) | 49 (94) | 49 (100) | 9 (100) | 2 (100) | 55 (100) | 3 (100) | 37 (97) | 1 (50) | 4 (80) | 9 (100) | ||||
| Histologic subtype | 0.002 | 0.009 | 0.183 | |||||||||||||||
| GT | 4 (9) | 2 (20) | 4 (12) | 8 (36) | 9 (17) | 7 (15) | 2 (22) | 0 (0) | 9 (17) | 0 (0) | 7 (18) | 0 (0) | 0 (0) | 2 (22) | ||||
| IT | 32 (71) | 2 (20) | 15 (44) | 6 (27) | 20 (39) | 33 (69) | 1 (11) | 1 (50) | 34 (63) | 2 (67) | 13 (34) | 1 (50) | 3 (60) | 2 (22) | ||||
| PT | 9 (20) | 4 (40) | 13 (38) | 3 (32) | 21 (40) | 7 (15) | 4 (44) | 1 (50) | 10 (19) | 1 (33) | 16 (42) | 1 (50) | 2 (40) | 3 (33) | ||||
| OT | 0 (0) | 2 (20) | 2 (6) | 1 (5) | 2 (4) | 1 (2) | 2 (22) | 0 (0) | 1 (2) | 0 (0) | 2 (5) | 0 (0) | 0 (0) | 2 (22) | ||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download