INTRODUCTION
The safety and efficacy of drugs, human derived products, or medical devices in clinical practice should be guaranteed. For physicians, the so-called ‘labelling (or licensing)’ is the one of the guidelines for the use of such drugs or medical devices based on evidence of safety and efficacy. However, in many clinical situations, drugs are more widely used than their approved indications, if they are deemed helpful to patients. In the pediatric population, many drugs used are ‘off-labeled’ or ‘unlicensed’ because labelling of most drugs is focused on their use in adults, and it is difficult to conduct clinical trials to assess the safety and efficacy of certain medicines with the approval of the regulatory bodies, in such vulnerable population.
1
Off-labeled/unlicensed use of medicines includes drug administration that varies from the label in terms of indications, doses, age, and routes of administration. A previous study reported that among 2,262 drug prescriptions administered to 624 children in five European hospitals, 1,036 (46%) were either off-labeled or unlicensed; and 67% of the patients (n = 421) received at least one such prescription.
2 Off-labeled or unlicensed prescription is inevitable; however, there are great concerns that the safety and efficacy of drugs depend on the experience of the clinician and are not scientifically approved. The off-labeled/unlicensed use of medications was implicated in adverse drug reactions in children; therefore, off-labeled/unlicensed drugs should be prescribed cautiously when the benefits exceed the risks.
3 However, to ensure the safety and efficacy of such medications in pediatric patients, efforts are needed at several levels such as governments, companies, health care providers, and parents.
45 It is important to assess the scale of off-labeled/unlicensed use of drugs or medical devices in children in real clinical practice. It could provide the information regarding the safety and efficacy of off-labeled/unlicensed use of drugs or medical devices.
In this study, we explored the off-labeled/unlicensed drug use in tertiary hospitals in children to assess the safety and efficacy of drugs and to obtain detailed information of such prescription use in children by reviewing the prescriptions and their efficacy and adverse drug reaction.
Go to :

METHODS
Study population
Pediatric patients who were admitted to the hospitals between January–December 2014 and received off-labeled/unlicensed drugs were enrolled. Patients who were aged > 18 years and lost to follow-up were excluded.
List of drugs
The off-labeled/unlicensed drugs were determined based on the information from the library of Ministry of Food and Drug Safety (MFDS) of Korea. We classified the use of drugs as off-labeled/unlicensed in following cases: dose or frequency was higher than approved, the medicine was not approved for the weight or age of the patient, the indication not approved, the route of administration not approved, and unlicensed/extemporaneous product used.
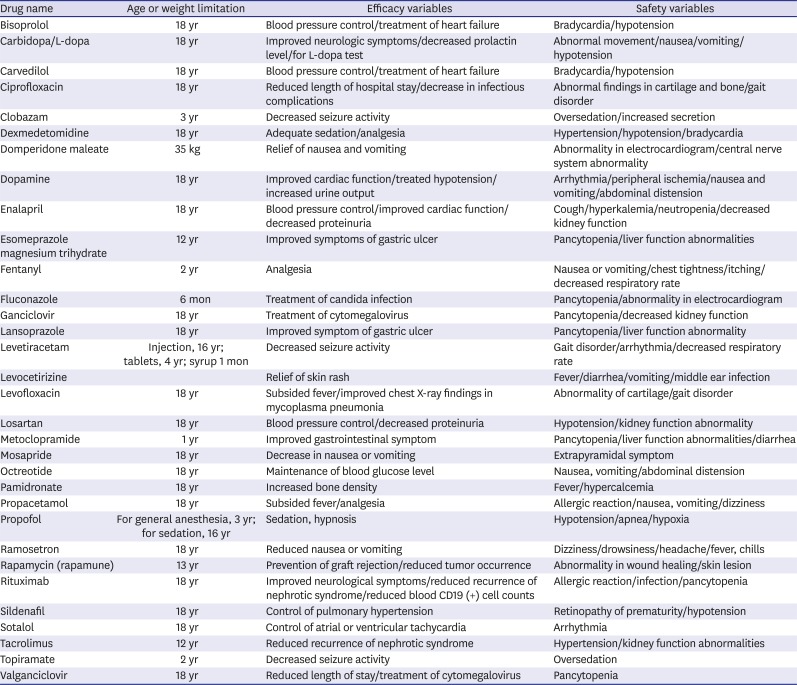
A large number of medicines are used in the clinical setting; we selected a list of drugs before reviewing the electronic medical records (EMRs). We performed a survey amongst the staffs of the tertiary pediatric hospital to identify the drugs that were mostly prescribed as off-labeled/unlicensed and needed to be changed to be labeled as soon as possible. We then chose 32 prescribed off-labeled/unlicensed drugs (
Table 1). The efficacy of each drug was assessed based on whether the expected effect of the drug was observed. To assess the safety, we evaluated not only the reported adverse drug reactions but also events that occurred immediately after the administration of such drugs. Adverse drug reaction was defined as follows, according to a previous report
6: an appreciably harmful or unpleasant reaction, resulting from drug administration.
Table 1
A list of 32 prescribed drugs and their information including efficacy and safety variables
|
Drug name |
Age or weight limitation |
Efficacy variables |
Safety variables |
|
Bisoprolol |
18 yr |
Blood pressure control/treatment of heart failure |
Bradycardia/hypotension |
|
Carbidopa/L-dopa |
18 yr |
Improved neurologic symptoms/decreased prolactin level/for L-dopa test |
Abnormal movement/nausea/vomiting/hypotension |
|
Carvedilol |
18 yr |
Blood pressure control/treatment of heart failure |
Bradycardia/hypotension |
|
Ciprofloxacin |
18 yr |
Reduced length of hospital stay/decrease in infectious complications |
Abnormal findings in cartilage and bone/gait disorder |
|
Clobazam |
3 yr |
Decreased seizure activity |
Oversedation/increased secretion |
|
Dexmedetomidine |
18 yr |
Adequate sedation/analgesia |
Hypertension/hypotension/bradycardia |
|
Domperidone maleate |
35 kg |
Relief of nausea and vomiting |
Abnormality in electrocardiogram/central nerve system abnormality |
|
Dopamine |
18 yr |
Improved cardiac function/treated hypotension/increased urine output |
Arrhythmia/peripheral ischemia/nausea and vomiting/abdominal distension |
|
Enalapril |
18 yr |
Blood pressure control/improved cardiac function/decreased proteinuria |
Cough/hyperkalemia/neutropenia/decreased kidney function |
|
Esomeprazole magnesium trihydrate |
12 yr |
Improved symptoms of gastric ulcer |
Pancytopenia/liver function abnormalities |
|
Fentanyl |
2 yr |
Analgesia |
Nausea or vomiting/chest tightness/itching/decreased respiratory rate |
|
Fluconazole |
6 mon |
Treatment of candida infection |
Pancytopenia/abnormality in electrocardiogram |
|
Ganciclovir |
18 yr |
Treatment of cytomegalovirus |
Pancytopenia/decreased kidney function |
|
Lansoprazole |
18 yr |
Improved symptom of gastric ulcer |
Pancytopenia/liver function abnormality |
|
Levetiracetam |
Injection, 16 yr; tablets, 4 yr; syrup 1 mon |
Decreased seizure activity |
Gait disorder/arrhythmia/decreased respiratory rate |
|
Levocetirizine |
|
Relief of skin rash |
Fever/diarrhea/vomiting/middle ear infection |
|
Levofloxacin |
18 yr |
Subsided fever/improved chest X-ray findings in mycoplasma pneumonia |
Abnormality of cartilage/gait disorder |
|
Losartan |
18 yr |
Blood pressure control/decreased proteinuria |
Hypotension/kidney function abnormality |
|
Metoclopramide |
1 yr |
Improved gastrointestinal symptom |
Pancytopenia/liver function abnormalities/diarrhea |
|
Mosapride |
18 yr |
Decrease in nausea or vomiting |
Extrapyramidal symptom |
|
Octreotide |
18 yr |
Maintenance of blood glucose level |
Nausea, vomiting/abdominal distension |
|
Pamidronate |
18 yr |
Increased bone density |
Fever/hypercalcemia |
|
Propacetamol |
18 yr |
Subsided fever/analgesia |
Allergic reaction/nausea, vomiting/dizziness |
|
Propofol |
For general anesthesia, 3 yr; for sedation, 16 yr |
Sedation, hypnosis |
Hypotension/apnea/hypoxia |
|
Ramosetron |
18 yr |
Reduced nausea or vomiting |
Dizziness/drowsiness/headache/fever, chills |
|
Rapamycin (rapamune) |
13 yr |
Prevention of graft rejection/reduced tumor occurrence |
Abnormality in wound healing/skin lesion |
|
Rituximab |
18 yr |
Improved neurological symptoms/reduced recurrence of nephrotic syndrome/reduced blood CD19 (+) cell counts |
Allergic reaction/infection/pancytopenia |
|
Sildenafil |
18 yr |
Control of pulmonary hypertension |
Retinopathy of prematurity/hypotension |
|
Sotalol |
18 yr |
Control of atrial or ventricular tachycardia |
Arrhythmia |
|
Tacrolimus |
12 yr |
Reduced recurrence of nephrotic syndrome |
Hypertension/kidney function abnormalities |
|
Topiramate |
2 yr |
Decreased seizure activity |
Oversedation |
|
Valganciclovir |
18 yr |
Reduced length of stay/treatment of cytomegalovirus |
Pancytopenia |

Review of the EMRs data and classification
We obtained a list of patients who received any of the 32 drugs between January–December 2014. Then, the investigators (doctors) at each site collected data including patient demographics, diagnoses, reasons for administration, routes of administration, and details of adverse drug reactions. Additionally, the mortality in the cohort was assessed. The age of the patients was categorized as follows: group 1, 0 days–1 month; group 2, 1–23 months; group 3, 2–11 years; and group 4, 12–18 years.
Data processing and statistical analysis
The primary outcomes were the efficacy and safety of the drugs, including mortality. And the secondary outcomes were the current statuses of the use of off-labeled/unlicensed drugs in the two centers. Descriptive analyses were performed to analyze the continuous variables. Normality of data was tested by Kolmogorov-Smirnov test. Categorical variables are expressed as numbers and percentages, and continuous variables as mean (standard deviation [SD]) or medians with the interquartile range. Student's t-test or Mann-Whitney rank-sum test was used to test the statistical significance of continuous data. Binary logistic regression analysis was performed to evaluate the association between parameters and adverse drug events. All analyses were performed using Excel 2013 for Windows and SPSS ver. 22 (IBM Corp., Armonk, NY, USA). A threshold of P < 0.05 was set to indicate statistical significance.
Ethics statement
This retrospective observational study was performed in two tertiary hospitals in the Republic of Korea, and approved by the Institutional Review Board of Seoul National University (H1603-086-749) and Severance Hospital (4-2016-0592). The requirement for written informed consent was waived.
Go to :

RESULTS
A total of 5,130 prescriptions in 2,779 patients with 1,325 males and 1,454 females at the two children's centers were identified. The age distribution of the patients were as follows: there were 176 (6.3%) patients in group 1, 735 in group 2, 890 (32.0%) in group 3, and 978 (35.2%) in group 4. Overall, 2,351 patients received two or more off-labeled/unlicensed drugs (84.6%) with a mean of 1.8 prescriptions per patient. Additionally, 1,136 prescriptions were from intensive care units, while 3,666 were from the wards, and 328 from the emergency departments.
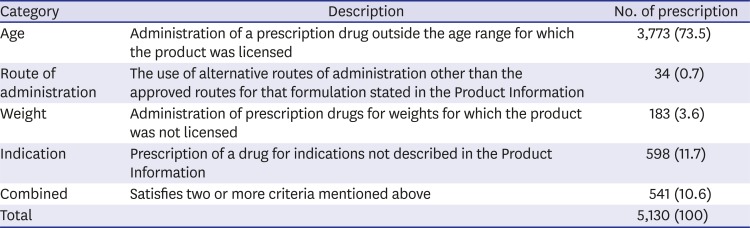
The drugs qualified as off-labeled/unlicensed in terms of age as the most common reason (73.5%), followed by indication (11.7%), weight (3.6%), and route of administration (0.7%) (
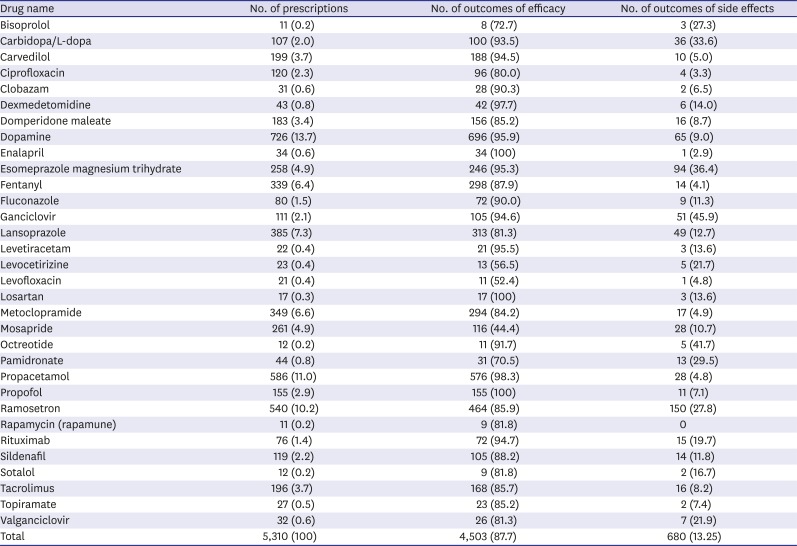
Table 2). The most commonly prescribed category of off-labeled/unlicensed drugs was antifungal agents (43.3%) followed by gastrointestinal drugs (23.0%), cardiovascular drugs (19.5%), analgesics (18.0%), and antiemetics (10.0%). The most frequently prescribed off-labeled/unlicensed drug as a single agent was dopamine (13.7%), followed by propacetamol (11.0%) and ramosetron (10.2%).
Table 3 shows the number of prescriptions and those demonstrating the efficacy and adverse drug reactions of each drug in the study cohort.
Table 2
The categories of off-labeled/unlicensed drugs used and their incidences
|
Category |
Description |
No. of prescription |
|
Age |
Administration of a prescription drug outside the age range for which the product was licensed |
3,773 (73.5) |
|
Route of administration |
The use of alternative routes of administration other than the approved routes for that formulation stated in the Product Information |
34 (0.7) |
|
Weight |
Administration of prescription drugs for weights for which the product was not licensed |
183 (3.6) |
|
Indication |
Prescription of a drug for indications not described in the Product Information |
598 (11.7) |
|
Combined |
Satisfies two or more criteria mentioned above |
541 (10.6) |
|
Total |
|
5,130 (100) |

Table 3
The number of prescriptions, and outcomes regarding efficacy and side effects of each drug in the cohort
|
Drug name |
No. of prescriptions |
No. of outcomes of efficacy |
No. of outcomes of side effects |
|
Bisoprolol |
11 (0.2) |
8 (72.7) |
3 (27.3) |
|
Carbidopa/L-dopa |
107 (2.0) |
100 (93.5) |
36 (33.6) |
|
Carvedilol |
199 (3.7) |
188 (94.5) |
10 (5.0) |
|
Ciprofloxacin |
120 (2.3) |
96 (80.0) |
4 (3.3) |
|
Clobazam |
31 (0.6) |
28 (90.3) |
2 (6.5) |
|
Dexmedetomidine |
43 (0.8) |
42 (97.7) |
6 (14.0) |
|
Domperidone maleate |
183 (3.4) |
156 (85.2) |
16 (8.7) |
|
Dopamine |
726 (13.7) |
696 (95.9) |
65 (9.0) |
|
Enalapril |
34 (0.6) |
34 (100) |
1 (2.9) |
|
Esomeprazole magnesium trihydrate |
258 (4.9) |
246 (95.3) |
94 (36.4) |
|
Fentanyl |
339 (6.4) |
298 (87.9) |
14 (4.1) |
|
Fluconazole |
80 (1.5) |
72 (90.0) |
9 (11.3) |
|
Ganciclovir |
111 (2.1) |
105 (94.6) |
51 (45.9) |
|
Lansoprazole |
385 (7.3) |
313 (81.3) |
49 (12.7) |
|
Levetiracetam |
22 (0.4) |
21 (95.5) |
3 (13.6) |
|
Levocetirizine |
23 (0.4) |
13 (56.5) |
5 (21.7) |
|
Levofloxacin |
21 (0.4) |
11 (52.4) |
1 (4.8) |
|
Losartan |
17 (0.3) |
17 (100) |
3 (13.6) |
|
Metoclopramide |
349 (6.6) |
294 (84.2) |
17 (4.9) |
|
Mosapride |
261 (4.9) |
116 (44.4) |
28 (10.7) |
|
Octreotide |
12 (0.2) |
11 (91.7) |
5 (41.7) |
|
Pamidronate |
44 (0.8) |
31 (70.5) |
13 (29.5) |
|
Propacetamol |
586 (11.0) |
576 (98.3) |
28 (4.8) |
|
Propofol |
155 (2.9) |
155 (100) |
11 (7.1) |
|
Ramosetron |
540 (10.2) |
464 (85.9) |
150 (27.8) |
|
Rapamycin (rapamune) |
11 (0.2) |
9 (81.8) |
0 |
|
Rituximab |
76 (1.4) |
72 (94.7) |
15 (19.7) |
|
Sildenafil |
119 (2.2) |
105 (88.2) |
14 (11.8) |
|
Sotalol |
12 (0.2) |
9 (81.8) |
2 (16.7) |
|
Tacrolimus |
196 (3.7) |
168 (85.7) |
16 (8.2) |
|
Topiramate |
27 (0.5) |
23 (85.2) |
2 (7.4) |
|
Valganciclovir |
32 (0.6) |
26 (81.3) |
7 (21.9) |
|
Total |
5,310 (100) |
4,503 (87.7) |
680 (13.25) |

With regard to the age of the patients, group 2 received the most number of off-label/unlicensed prescriptions (32.9%), followed by group 3 (31.1%), group 4 (29.4%), and group 1 (5.5%). In terms of the wards, 71.2% of prescriptions were from the general ward, while 22.1% were from intensive care units, and 6.4% from emergency departments. Regarding out-patient department, most of the data were excluded because the data to assess the safety and efficacy were not available. Therefore, only a small proportion of prescriptions (0.8%) appear to be from the out-patient departments.
A total of 523 patient developed adverse drug reactions (18.8%). The number of prescriptions was significantly higher in children with adverse drug reactions than it was in those without adverse drug reactions (2.8 vs. 1.5; 95% confidence interval [CI], 1.0–1.5; P < 0.001]). The mean age was lower in patients with adverse drug reactions that it was in those without, but it was not statistically significant (7.5 vs. 8.0 years; 95% CI, −0.3–0.8 years; P = 0.08).
Binary logistic regression determined that the number of prescribed off-label/unlicensed medicines and the age at prescription were independently associated with subsequent adverse drug events (odds ratio [OR], 1.55; 95% CI, 1.44–1.65; P < 0.001 for the number of medicines; OR, 1.1; 95% CI, 1.0–1.2; P = 0.034 for age).
Of the 2,779 patients who received off-labeled/unlicensed prescriptions, 112 patients died during the follow-up period. None of the deaths were associated with the use of off-labeled/unlicensed drugs, but they appeared to be associated with the underlying diseases. The primary diagnoses in these patients were categorized as follows: hemato-oncologic diseases (n = 37, 33%), congenital heart diseases (n = 19, 17%), airway and respiratory diseases (n = 16, 14.3%), gastrointestinal diseases (n = 10, 8.9%), immunologic diseases (n = 5, 4.5%), preterm births (n = 5, 4.5%), renal and urinary tract diseases (n = 4, 3.6%), cardiovascular diseases other than congenital heart diseases (n = 3, 2.7%), cerebrovascular diseases (n = 3, 2.7%), sepsis (n = 3, 2.7%), neuromuscular diseases (n = 2, 1.8%), hormonal or metabolic diseases (n = 2, 1.8%), brain death (n = 2, 1.8%), and orthopedic diseases (n = 1, 0.9%).
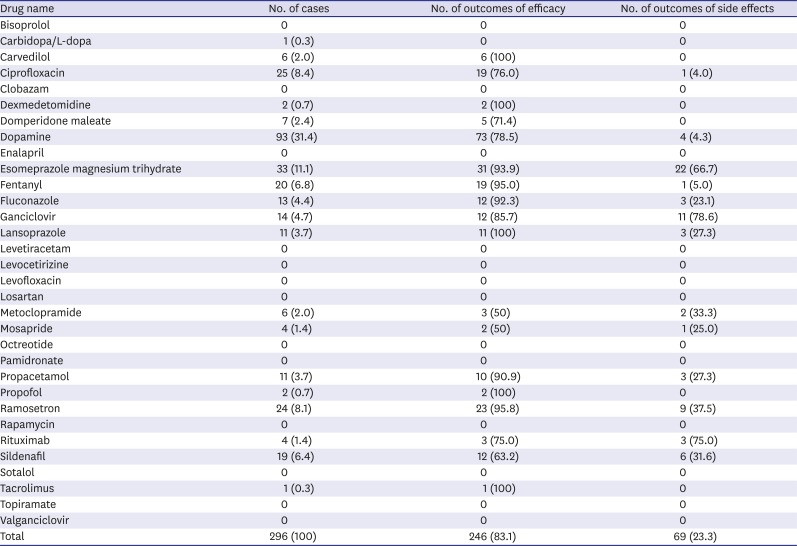
A total of 296 unlabeled prescriptions were identified in these patients (an average of 2.6 prescriptions per patient). The most frequently prescribed off-labeled/unlicensed drugs were dopamine (31.4%), esomeprazole magnesium trihydrate (11.1%), ciprofloxacin (8.4%), and ramosetron (8.1%). The most frequent adverse drug reactions were hematocytopenia (n = 28), liver function abnormalities (n = 20), fever (n = 9), hypotension (n = 5), vomiting (n = 3), and headache (n = 2). The number of prescriptions, and the number of cases with outcomes of efficacy and adverse drug reactions are shown in
Table 4.
Table 4
The number of prescriptions, and outcomes of efficacy and side effects of each drug along with mortality
|
Drug name |
No. of cases |
No. of outcomes of efficacy |
No. of outcomes of side effects |
|
Bisoprolol |
0 |
0 |
0 |
|
Carbidopa/L-dopa |
1 (0.3) |
0 |
0 |
|
Carvedilol |
6 (2.0) |
6 (100) |
0 |
|
Ciprofloxacin |
25 (8.4) |
19 (76.0) |
1 (4.0) |
|
Clobazam |
0 |
0 |
0 |
|
Dexmedetomidine |
2 (0.7) |
2 (100) |
0 |
|
Domperidone maleate |
7 (2.4) |
5 (71.4) |
0 |
|
Dopamine |
93 (31.4) |
73 (78.5) |
4 (4.3) |
|
Enalapril |
0 |
0 |
0 |
|
Esomeprazole magnesium trihydrate |
33 (11.1) |
31 (93.9) |
22 (66.7) |
|
Fentanyl |
20 (6.8) |
19 (95.0) |
1 (5.0) |
|
Fluconazole |
13 (4.4) |
12 (92.3) |
3 (23.1) |
|
Ganciclovir |
14 (4.7) |
12 (85.7) |
11 (78.6) |
|
Lansoprazole |
11 (3.7) |
11 (100) |
3 (27.3) |
|
Levetiracetam |
0 |
0 |
0 |
|
Levocetirizine |
0 |
0 |
0 |
|
Levofloxacin |
0 |
0 |
0 |
|
Losartan |
0 |
0 |
0 |
|
Metoclopramide |
6 (2.0) |
3 (50) |
2 (33.3) |
|
Mosapride |
4 (1.4) |
2 (50) |
1 (25.0) |
|
Octreotide |
0 |
0 |
0 |
|
Pamidronate |
0 |
0 |
0 |
|
Propacetamol |
11 (3.7) |
10 (90.9) |
3 (27.3) |
|
Propofol |
2 (0.7) |
2 (100) |
0 |
|
Ramosetron |
24 (8.1) |
23 (95.8) |
9 (37.5) |
|
Rapamycin |
0 |
0 |
0 |
|
Rituximab |
4 (1.4) |
3 (75.0) |
3 (75.0) |
|
Sildenafil |
19 (6.4) |
12 (63.2) |
6 (31.6) |
|
Sotalol |
0 |
0 |
0 |
|
Tacrolimus |
1 (0.3) |
1 (100) |
0 |
|
Topiramate |
0 |
0 |
0 |
|
Valganciclovir |
0 |
0 |
0 |
|
Total |
296 (100) |
246 (83.1) |
69 (23.3) |

Go to :

DISCUSSION
This retrospective observational study investigated the use of off-labeled/unlicensed medicines in two tertiary pediatric hospitals in the Republic of Korea. On an average, approximately two off-labeled/unlicensed drugs were prescribed per patient, and the incidence of off-labeled/unlicensed prescription was higher in younger patients. The children who died had higher number of off-labeled/unlicensed prescriptions than did the total cohort.
Off-labeled/unlicensed administration of drugs in pediatric centers is common in tertiary hospitals. Several studies have investigated the use of off-label or unlicensed medicines in pediatric centers.
78910 According to one study, the reported off-labeled or unlicensed prescription rates ranged from 11%–80% in different clinical settings,
11 while another study reported that more than 90% of children received off-label/unlicensed medicines in Norway.
7 In contrast to previous studies, we reviewed only the off-label prescriptions and did not include the children without off-labeled/unlicensed prescription. Therefore, we could not assess the incidence of off-label prescriptions out of the total prescriptions.
In our study, the primary reasons for off-labeled/unlicensed prescriptions were age and indications, which was a similar finding of a previous systematic review.
12 On the other hand, other studies reported that the route of administration was the most common reason for off-labeled/unlicensed prescriptions,
713 for example, buccal administration of sedatives and analgesics, and administration of drugs via enteral feeding tube. We believe that the differences between our study and previous reports regarding the reasons might be the differences in the route of administration in children. For example, according to Lindell-Osuagwu et al.,
13 buccal administration of sedatives and analgesics was the primary reason for off-labeled/unlicensed prescriptions. In the centers we investigated, buccal or nasal administration of drugs in children were rare. Additionally, off-label handling, such as tablet splitting or tablet/capsule dispersion, was typical in children. However, this type of off-label handling might be missing from EMRs.
7
Of the pediatric patients who received off-labeled/unlicensed prescriptions, the children of the youngest age group received the highest number of such prescriptions in the present study. Our results were in accordance with those of previous studies,
1114 demonstrating that lower the age of a child, the more likely doctors are to use off-label or unlicensed prescriptions. A more recent study also reported that most off-label prescriptions occurred in infants and children, with percentage > 60%.
10 On the other hand, one report from a French hospital showed that adolescents (12–18-year-old) and children (2–11-year-old) received the highest number of off-label/unlicensed prescriptions (46.8% and 45%, respectively).
9 However, in that study, the number of neonates and infants assessed was very small.
As single agents, dopamine and propacetamol were the most frequently prescribed medicines in this study. Dopamine has been used in the pediatric population for over 40 years.
15 However, according to drug information provided by the Korea MFDS, its safety and efficacy in the pediatric population has not been investigated, which was similar to the drug information provided by the US Food and Drug Administration and European Medicine Agency. Therefore, we included dopamine in the list of off-labeled/unlicensed drugs in the present study. Additionally, the use of intravenous propacetamol in neonates and children has gained popularity,
1617 and it has been widely used for postoperative pain control and as an antipyretic in Korea. In US, intravenous acetaminophen is approved in children aged > 2 years. In European countries, it has been approved in children immediately after birth
18 and is most frequently used for analgesia in the pediatric wards in European countries.
2 Therefore, we believe that it is necessary to reconsider the authorization of drugs that have been used for a long time, especially when the prescription is for therapeutic use.
In this study, we intended to evaluate adverse drug reactions of the off-labeled prescriptions. There have been several reports regarding the risk assessment of off-labeled/unlicensed medicines in pediatric patients.
3192021 Two review articles investigated the rate of adverse effects associated with the use of off-label/unlicensed medicines and found that it varied from 17%–60%.
2122 Other prospective studies found that the ORs of an off-labeled/unlicensed medicine being implicated in an adverse drug reaction compared with an authorized medicine, was from 1.67–2.84 in children.
31920 A study by Bellis et al.
19 demonstrated that the number of off-labeled/unlicensed medicines was not a predictor of the risk of adverse drug reactions. On the other hand, Saiyed et al.
3 reported that the hazard of an adverse drug reaction significantly increased with the number of off-label medicines prescribed (hazard ratio of 1.28), which was a similar rate as our results. Many off-labeled drugs in children are prescribed in combination; therefore, clinicians should closely monitor the responses of children administration of such drugs.
Along with the number of off-labeled/unlicensed medicines, age was also a risk factor of adverse drug reactions in the present study. The OR was 1.1, which was similar to that reported by a previous prospective study performed in the UK (OR, 1.04).
19 We assume that the off-labeled/unlicensed drugs might be overused in terms of frequency or doses in older children or adolescents, whereas clinicians should be more careful when treating infants or neonates. However, careful interpretation is required for this result. This was a retrospective study; therefore, the outcomes may be affected by confounding factors after drug administration, such as the severity of illness or conditions that require surgery. For example, increasing age was a risk factor for adverse drug events in oncologic patients, but not in non-oncologic patients.
19 Additionally, there was difficulty in distinguishing the symptoms due to the underlying pathologies and adverse drug reactions in some patients.
Although children with mortality received a relatively greater number of off-labeled/unlicensed medicines than children without mortality (2.6 vs. 1.6 per pediatric patient), the authors could not determine a definite association between the use of medicines and death because the children had wide range of disease conditions at various stages. In the present study, the hemato-oncologic disease was the most common diagnosis in children with mortality. This result is similar to a recent study by Kim et al.,
23 demonstrating that malignancy was the most common cause of death in children with complex chronic conditions in Korea. Disease severity might be related to the amount of medication used, including off-label medicine use. Further study should focus on the efficacy and safety of off-label/unlicensed medicine usage within a similar patient population.
Our study has several limitations. First, we did not evaluate the total prescriptions of all medicines in the same period; therefore, the incidence of off-label prescriptions could not be calculated. Second, the efficacy and safety of medicines prescribed to outpatients could not be evaluated well due to a lack of EMR regarding the follow-up information. Third, the drugs prescribed solely in children who required chemotherapy could not be evaluated; it was difficult to determine the adverse drug reactions of each anticancer drug because many off-label drugs were administered simultaneously. Additionally, the short-term response after anticancer drug administration was hard to predict in children who underwent chemotherapy. The anticancer medications were mostly prescribed for treatment, i.e., there were no other drug options for those patients. Finally, the implications of off-labeled/unlicensed use might be more important when considering the long-term adverse events, as well as immediate ones, especially in children. For example, the overuse or underuse of cardiovascular drugs such as dopamine and milrinone may result in hemodynamic instability and related sequelae.
24 However, the safety assessment was mainly focused on the short-term adverse drug events in our study.
In summary, this study demonstrated the off-labeled/unlicensed drug use in children in tertiary hospitals in Korea and evaluated the safety of drugs retrospectively. Children continue to receive medicines that were not authorized in terms of age, weight, indication, or route of administration. Therefore, many old products require re-assessment of authorization. Adverse drug reactions are still associated with the use of these drugs. Additionally, the labelling of drugs differs between countries. Therefore, further prospective clinical studies are needed to confirm the efficacy and safety of drugs in the pediatric population. Additionally, efforts by pediatricians and regulatory authorities are needed to improve pediatric drug labelling.
Go to :









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download